Of cactuses and bromeliads
Of cactuses and bromeliads
By Lauren Trotta
If asked to summon a plant, what is the first form conjured by your mind’s eye? A tree, with a towering trunk, topped with a tufted halo of green? Or maybe a dainty stem springing forth from a patch of earth with leaves held wide to soak up the sun. What about a cactus with fleshy ribs and a constellation of woody spines or an air plant with a spiral of mossy gray leaves that seem to have broken free from the substrate required by most of their botanical brethren? These unique growth forms are a result of two disparate but similar evolutionary trajectories that allowed these lineages to thrive in arid spaces, from open sandy habitats, to a forest canopy, to the windowsill or bookshelf in your home where watering can be, well, infrequent.
For my final post as a UFBI fellow I wanted to share two stories of, what I consider, fascinating, botanical oddballs in Florida. Bromeliads and cactuses, while not particularly closely related in an evolutionary context, represent two lineages of Neotropical plants (meaning they originated in and are primarily distributed in at the tropics of the South, Central, and North America) that have made their way to Florida based on similar adaptations and have persisted here using idiosyncratic life history strategies. It is their uniqueness that has ultimately made them highly vulnerable to the multifaceted intrusions of anthropogenic change.
By Land, By Sea: Living on the Edge
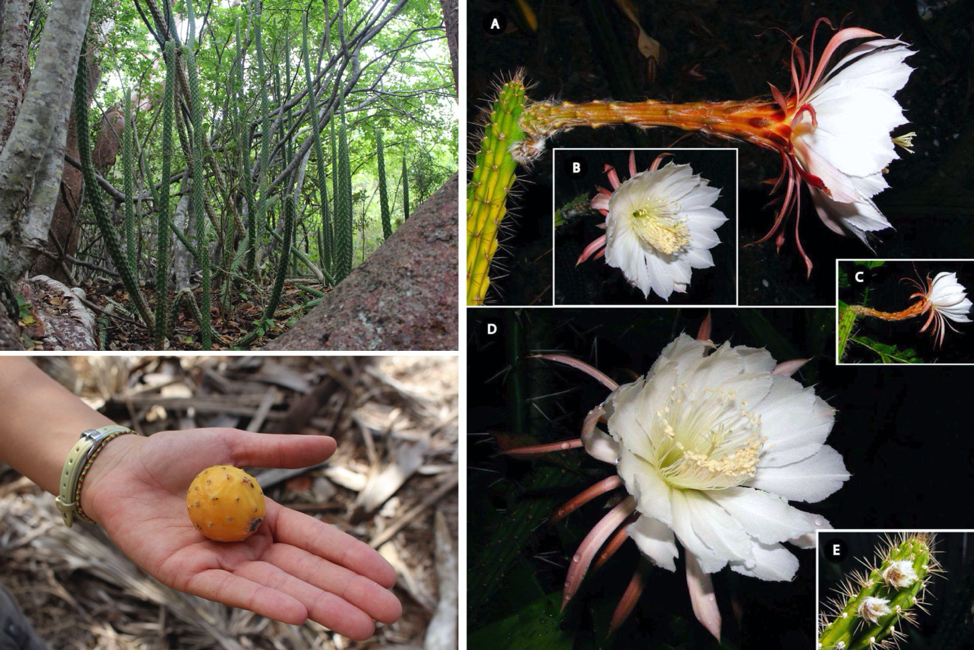
Perched just beyond the high tide mark of Florida’s coasts lie some of the most endangered habitats in the state. Historically, coastal habitats would have ranged from open scrub, thicketed coastal berms, and humid rockland and maritime hammocks in the south and east to sandy or shelly coastal strands and dense maritime hammocks on the west coast. Today, these habitats remain as subdivided fragments squeezed between human development of beachside residences or sea walls, and the parcels that do remain are posed to be the first to feel the effects of sea level rise (Bradley et al., 2002, 2004; Stys et al., 2017). Here, growing in the goldilocks-esque spots that are not to shaded and not to bright, you can find two different, closely related cactuses: the Indian River prickly-apple (Harrisia fragrans) and West coast prickly-apple (Harrisia aboriginum) (Franck, 2016).

Like long, succulent pencils, these cactuses can grow to 4m tall and are adorned with clusters of darkly-tipped tawny spines that jut in every direction reminiscent of a miniature pin cushion. Their large, luxurious flowers have a long neck topped with a ruffle of white petals that open only for one night1 after which H. aboriginum produces spiky yellowish fruit the size and color of an underripe tomato, while the fruits of H. fragrans are a much brighter orange-red color. These two species are thought to be narrow endemics (having arisen and are now found in only a small area) and represent the northern most extent of this genus which arose in the Andes 3-7 million years ago and proceeded to spread to Brazil before island hopping through the West Indies and arriving in Florida (Franck et al., 2013a, 2013b; Franck, 2016). Similar to many other cactus species, the reduction of leaves into spines and the expansion of stems in to fleshy, water storing structures are adaptations to survive in arid environments (Guerrero et al, 2019). Further, the green stems of these species are able to partake in a specialized form of photosynthesis called CAM, which allows the plant to avoid desiccation by breathing in the CO2 necessary to perform photosynthesis at night and storing it for later use (Franck, 2016). In terms of reproduction, these species are flexible; they can fruit after outcrossing or self-pollination and spread vegetatively from the breaking of branches (Franck, 2016).
The adaptations that facilitated expansion of Harrisia into arid environments likely resulted in the distribution of these species expanding and contracting to match the dominant environmental conditions of the day (Franck, 2016). Because both H. fragrans and H. aboriginum require “goldilocks” light levels while being embedded in a dynamic matrix of species, it seems likely that, historically, these species would have leap-frogged around the landscape to find their optimal habitat. Further, while some of the habitats that host Harrisia are fire adapted, Harrisia itself cannot survive being burned. The long-term survival of these Harrisia species depended on diversifying their portfolio of locations so that when conditions became untenable for plants in one area other populations could persist in another area until conditions changed once again.
By Air: Attack of the Weevil
While we generally think of forests in terms of trees, a recent study evaluated this bias. They found that our tree-based sampling mistakes the forest for the trees and severely underestimates the biodiversity found in the canopy (Spicer et al, 2020). Other growth forms like epiphytes (plants that grow on other plants) are much more difficult to access, but they contribute heavily to the diversity of forest ecosystems and serve substantial roles in facilitating the persistence of other interconnected species.
Subtropical Florida has an iconic and ecologically important epiphyte community of bromeliads, ferns, and orchids. There are 16 native species of bromeliads in Florida, with the majority of the diversity belonging to the genus Tillandsia (Frank and Cave, 2005). This lineage, like to Harrisia, arose in the Andes before making its way to Western Mesoamerica and spreading through the West Indies to Florida (Pinzón et al. 2016, 2019). The largest species of this genus in Florida, the Giant Airplant (Tillandsia utriculata2), can grow up to a meter in diameter and their tanks can hold up to 1.3L of water (Cooper et al, 2014).
Much like a dry, hot desert, the tree canopies where T. utriculata live can be a very difficult place to inhabit, and bromeliad species have evolved specialized structures and life strategies that allow them to survive these arid conditions (Benzing, 2000). The first adaptation is a shift to a rosette growth form where leaves grow in a circular swirl around the center of the plant. In some species, this rosette creates a vase-like tank that captures and holds rainwater creating its own tiny ecosystem.3 A second adaption, the evolution of trichomes, or hairs, that look like a microscopic balloon or umbrella protruding from the surface of the leaf allow Tillandsia species to absorb water and nutrients directly from the air or from their tank (Benzing, 2000; Palma-Silva et al., 2016). Finally, CAM photosynthesis has evolved multiple times in the bromeliad family, including in Tillandsia species (Males et al., 2017). In combination, these adaptions allow T. utriculata and other Tillandsia speciesto live as atmospheric epiphytes where they act as ecosystem engineers by creating canopy structure and microhabitats that multitudes of other species depend on. A study of the macroscopic invertebrate species diversity in some of these tank communities found dozens of species ranging from ants and flies to millipedes and spiders, including a handful species that were endemic to the tank or previously undescribed (Frank et al., 2004). These tank habitats are also known to be source of water or food for many vertebrate species that can access the canopy like lizards, frogs, and birds.
For T. utriculata, their size gave them an outsized ecosystem role in the canopy until they were attacked and decimated by an invasive species, the Mexican Bromeliad Weevil (Metamasius callizona). In 1989, this invasive species was discovered to have been imported with a shipment of ornamental bromeliads and had quickly established in Florida’s native bromeliad populations. As an adult, the Mexican Bromeliad Weevil can eat the leaves of bromeliads, but the majority of the damage they inflict occurs by laying their eggs in the tank habitat. The developing weevil larvae burrow into and feast on the fleshy innards of the bromeliad beneath the tank, eventually killing the plant. This weevil is known to attack multiple native bromeliad speices4, but they have been most devastating to T. utriculata. One of main growth and reproductive strategies for bromeliads is called pupping – which occurs when a large plant creates a connected vegetative offshoot. Instead of creating these miniature clones, the T. utriculata plants found in Florida5 mature slowly (10+ years), bloom once, and die. As the invasive weevil has spread it has preferentially attacked large, old T. utriculata resulting in a massive reduction in the number of these plants that are able to bloom and establish the next generation, with accompanying losses of structure and habitat in forest canopies (Cooper et al., 2014).6

The formation of these diverse epiphyte communities generally occurred in the canopies of forested wetlands. The constant or intermittent inundation of these habitats facilitated higher air moisture levels required by atmospheric air plants to survive. Historically, crowds of orchids, ferns, and bromeliads clustered around water sources creating their own protective cocoon of relative warmth and humidity which allowed them to persist through freezes.7 These unique communities required relative stability and considerable time to assemble and accumulate not only the broad diversity of epiphytic flora, but all of their associated fauna.
Constant Change
Florida has been a highly variable landscape for millennia with even relatively recent shifts in sea level rise and aridity (Webb, 1990). As a result, lineages like cactuses and bromeliads were able to leverage their drought adaptations to expand their ranges when environmental conditions allowed and retract their ranges when the tide turned. One of the main conservation issues for these species is the mismatch between the rate anthropogenic climate change and the rate at which these species successfully adapt, regardless of whether the pressure is from rising seas or hungry invasive species. Compounding this incongruence in rates of change is the vast and ever-increasing reduction in potential habitat for these species to move to (Stys et al., 2017).
For Harrisia, human developmental pressures have resulted in less and less potential natural area for these species to move to. In some privately owned sites where these species do remain, they are now subject to the landscaping whims of the human owners (Bradley et al., 2004).8 Further, the requisite habitats for these species have diverged from their natural successional trajectories due to changes in fire, invasive species, and stochastic events like direct impact or storm surges from hurricanes (Bradley et al, 2002). Of the two species, H. aboriginum has both a more restricted range and is lower lying (0-5m above sea level) than H. fragrans (0-10m above sea level) making its vulnerability to climate change more imminent.
In the future, aquatic habitats, like the forested wetlands epiphytes depend on, are going to become more arid (Overpeck and Udall, 2020; Stys et al., 2017). Further, anthropogenetic climate change is also expected to lead to more severe shifts in temperature between hot to cold which will affect epiphytes held aloft in the canopy. Since the introduction of the Mexican Bromeliad Weevil the warm, humid microclimate once held stable by densely packed, water-filled species like T. utriculata has been alter substantially. The epiphytes that remain and all of the species that depend on them are now more vulnerable to drastic shifts in temperature and humidity that will come with more extreme weather events. As Florida and its biodiverse inhabitants face a future increasingly impacted by anthropogenic climate change adopting a multifaceted understanding of diversity – not just who is there, but why and how – will help us plan for the future.
Follow Lauren on Twitter.
Check out blogs from other UFBI Fellows.
FOOTNOTES
1 As Roger Hammer, Florida botanist extraordinaire relates in his Complete Guide to Wildflowers: Indian river prickly-apple (Harrisia fragrans) “flowers open several hours after sunset and close long before sunrise, so it takes true dedication, or total insanity, to be in its habitat at night with dense warms of salt marsh mosquitoes” (Hammer, 2018; p. 291)
2 The specific epithet of Tillandsia utriculata originates from the word utriculus which means “small water bag” (Pinzón et al., 2019)
3 Phytolemata are structures created by non-aquatic plants that capture water. Other notable examples of plants with these structures in Florida are pitcher plants in the genus Sarracenia [LT1] whose modified, cup-like leaves capture rain water.
4 Other native tank bromeliads include Guzmania monostacha and Tillandsia fascicualta, which are known to be attacked by the weevil, and three species of Catopsis which seem likely to be attacked by the weevil but there have been no observed instances, probably due to the rarity of these species (Frank and Cave, 2005)
5 Tillandsia utriculata has a large range outside of Florida from northern South America into Central America and across the Caribbean basin. The growth habit, flowers, and reproductive strategy of this species are highly variable across its range – only the individuals in Florida flower once and die (Pinzón et al., 2019).
6 For some excellent photos of Florida’s native bromeliads as well more on the Mexican Bromeliad Weevil check out a slideshow [LT2] from the lead author of the Cooper et al., 2014 study.
7 You can get a sense of what the epiphyte community used to look like in South Florida from these iconic photos taken in Cigar Orchid Pond[LT3] , Fakahatchee[LT4] Strand[LT5] , and Loosescrew Swamp Sanctuary[LT6] captured by famed Florida photographer, Clyde Butcher.
8 The Keys Tree-Cactus [LT7] (Pilosocereus robinii) is another Florida cactus that is facing many of the same challenges as Harrisia. With a range spanning from Cuba to the Florida Keys this species can grow up to 10m tall and is associated with increasingly rare habitat types in the Keys (like cactus hammocks) that have been diminished by human development and will be some of the first habitats impacted by sea level rise.
REFERENCES
Benzing, D.H., 2000. Bromeliaceae Profile of an Adaptive Radiation. Cam- bridgeUniversityPress, United Kingdom.
Bradley, Keith A, Janice Duquesnel, Steven W Woodmansee, Anthony L Koop, and George D Gann. 2002. “Population Status and Demography of the Endangered Harrisia Fragrans (Cactaceae) at Savannas Preserve State Park,” 1-36.
Bradley, Keith A, Steven W Woodmansee, and George D Gann. 2004. “Status Survey of Aboriginal Pricklyapples, Harrisia aboriginum Small Ex Britton & Rose, in Southwestern Florida,”1-40.
Cooper, Teresa M., J. Howard Frank, and Ronald D. Cave. 2014. “Loss of Phytotelmata Due to an Invasive Bromeliad-Eating Weevil and Its Potential Effects on Faunal Diversity and Biogeochemical Cycles.” Acta Oecologica 54 (January): 51–56. https://doi.org/10.1016/j.actao.2013.01.016.
Franck, Alan R. 2016. “Monograph of Harrisia (Cactaceae).” Phytoneuron 85: 1–159.
Franck, Alan R., Bruce J. Cochrane, and James R. Garey. 2013a. “Phylogeny, Biogeography, and Infrageneric Classification of Harrisia (Cactaceae).” Systematic Botany 38 (1): 210–23. https://doi.org/10.1600/036364413X662105.
Franck, Alan R., Bruce J. Cochrane, and James R. Garey. 2013. “Relationships and Dispersal of the Caribbean Species of Harrisia (Sect. Harrisia; Cactaceae) Using AFLPs and Seven DNA Regions.” Taxon 62 (3): 486–97. https://doi.org/10.12705/623.5.
Frank, Howard, and Ronald Cave. 2005. “Metamasius Callizona Is Destroying Florida’s Native Bromeliads.” Second International Symposium on Biological Control of Arthropods: 91-101.
Frank, J. H., S. Sreenivasan, P. J. Benshoff, M. A. Deyrup, G. B. Edwards, S. E. Halbert, A. B. Hamon, et al. 2004. “Invertebrate animals extracted from native Tillandsia (Bromeliales: Bromeliaceae) in Sarasota county, Florida.” Florida Entomologist 87 (2): 176–85. https://doi.org/10.1653/0015-4040(2004)087[0176:IAEFNT]2.0.CO;2.
Guerrero, Pablo C, Lucas C Majure, Amelia Cornejo-Romero, and Tania Hernández-Hernández. 2019. “Phylogenetic Relationships and Evolutionary Trends in the Cactus Family.” Journal of Heredity 110 (1): 4–21. https://doi.org/10.1093/jhered/esy064.
Hammer, R. L. 2018. Complete Guide to Florida Wildflowers: Over 600 Wildflowers of the Sunshine State Including National Parks, Forests, Preserves, and More Than 160 State Parks. United States: Falcon Guides.
Males, Jamie, and Howard Griffiths. 2017. “Functional Types in the Bromeliaceae: Relationships with Drought‐resistance Traits and Bioclimatic Distributions.” Edited by Rafael Oliveira. Functional Ecology 31 (10): 1868–80. https://doi.org/10.1111/1365-2435.12900.
Overpeck, Jonathan T., and Bradley Udall. 2020. “Climate Change and the Aridification of North America.” Proceedings of the National Academy of Sciences 117 (22): 11856–58. https://doi.org/10.1073/pnas.2006323117.
Pinzón, Juan P., Ivón M. Ramírez-Morillo, Germán Carnevali, Michael H. J. Barfuss, Walter Till, Juan Tun, and Juan J. Ortiz-Díaz. 2016. “Phylogenetics and Evolution of the Tillandsia Utriculata Complex (Bromeliaceae, Tillandsioideae) Inferred from Three Plastid DNA Markers and the ETS of the Nuclear Ribosomal DNA.” Botanical Journal of the Linnean Society 181 (3): 362–90. https://doi.org/10.1111/boj.12425.
Pinzón, Juan P., Ivón M. Ramírez-Morillo, Germán Carnevali, Walter Till, Derek Butcher, and Juan J. Ortiz-Díaz. 2019. “Taxonomic Treatment of the Tillandsia Utriculata Complex (Bromeliaceae).” Annals of the Missouri Botanical Garden 104 (2): 262–323. https://doi.org/10.3417/2019315.
Spicer, Michelle Elise, Hannah Mellor, and Walter P. Carson. 2020. “Seeing beyond the Trees: A Comparison of Tropical and Temperate Plant Growth Forms and Their Vertical Distribution.” Ecology 101 (4). https://doi.org/10.1002/ecy.2974.
Stys, Beth, Tammy Foster, Mariana Fuentes, Bob Glazer, Kimberly Karish, Natalie Montero, and Joshua Reece. 2017. “Climate Change Impacts on Florida’s Biodiversity and Ecology.” In Florida’s Climate: Changes, Variations, & Impacts, by Florida Climate Institute. Florida Climate Institute. https://doi.org/10.17125/fci2017.ch12.
Webb, S. David. 1990. “Historical Biogeography.” In Ecosystems of Florida, edited by Ronald L. Myers and John J. Ewel, 70–100.
ACKNOWLEDGEMENTS
I would like to thank Dr. Sally Chambers, research botanist at Marie Selby Botanical Gardens[LT8] , for her help formulating this piece. Her research program includes improving our understanding of the ecology, evolutionary biology, demography, and population genetics for Harrisia aboriginum and Tillandsia utriculata. You can find out more about her work on ResearchGate[LT9] and GoogleSchola[LT10] r.
[LT1]LINK: https://edis.ifas.ufl.edu/uw378
[LT2]LINK: https://conference.ifas.ufl.edu/geer/past/geer2010/Presentations/Tuesday/Royal%20Palm%203/am/0920%20T%20cooper.pdf
[LT3]LINK: https://clydebutcher.com/s/new-cats/united-states/usa-florida/cigar-orchid-pond-south-fl/
[LT4]LINK: https://clydebutcher.com/s/new-cats/united-states/usa-florida/fakahatchee-3-south-west-fl/
[LT5]LINK: https://clydebutcher.com/s/new-cats/united-states/usa-florida/fakahatchee-strand-5-south-west-fl/
[LT6]LINK: https://clydebutcher.com/s/photographs/florida-collection/national-parks/big-cypress-national-preserve/loosescrew-swamp-sanctuary-2-south-fl/
[LT7]LINK: https://www.fws.gov/verobeach/MSRPPDFs/KeyCactus.PDF
[LT8]https://selby.org
[LT9]LINK: https://www.researchgate.net/profile/Sally-Chambers-2
[LT10]LINK:https://scholar.google.com/citations?hl=en&user=U4q7ToAAAAAJ&view_op=list_works&sortby=pubdate


