2022-2023 BLOG POST
2022-2023 BLOG POST
Kate Davis: You Are What You Drink…
Abigail Uehling: A Unique Reference Library
Maria Cortez: Shifting Our Mindsets Towards Biocultural Diversity Conservation
Brittany Cummings: It’s the Little Things…
Barry Sosa: Microbes: The Unseen Environmental Workforce
Mitch Walters: To App or Not to App … That is the Question
Natalia Uribe-Castaneda: Socio-ecological Frameworks … Coral Reef Restoration
Maria Cortez: Serra do Cipó National Park
Brittany Cummings: The Hunt for Marine Invertebrates
Abigail Uehling: Endless Invertebrate Finds in Oman’s Diverse Water
Barry Sosa: The True Value of Science Outreach
Natalia Uribe-Castaneda: Science Communication
Brittany Cummings: Coloring Outside the Lines … Part 1
Barry Sosa: Upper Florida Aquifer Illuminates the Search for Life Beyond Earth
Natalia Uribe-Castaneda: Community engagement in Natural Resources Management
Maria Cortez: Weaving Women, Science, Creativity, and a Better Education
Brittany Cummings: Coloring Outside the Lines … Part 2
Barry Sosa: Challenges and Outcomes of Designing an Outreach Project: My Experience as a UFBI Fellow
Abigail Uehling: Star Predators
Kate Davis, MS
You are What you Drink: table isotopes and the fight against the illegal wildlife trade
The chemical elements that make up water, hydrogen (H) and oxygen (O), come in different varieties due to their molecular weight. These varieties, for example deuterium, the heavier variety of hydrogen, are known as isotopes. Isotopes are considered stable if they do not decay into other elements. The ratio of light to heavy stable isotopes in water follows predictable patterns in global precipitation. Rain that has just come in off the coast will have more heavy isotopes than a storm that has traveled inland. These patterns also exist across latitudes.
As organisms uptake the water in their ecosystems through drinking, respiration, and diet, isotopes are incorporated into their developing tissues, leaving a signature of where they’ve been. This principle has been used in human forensic analysis of remains of unknown origin by taking hair samples and looking at the isotope values along the length of the shaft. This can also be applied to items from the illegal wildlife trade, such as rhino horn and pangolin scales, when confiscated in a seizure, allowing conservationists and wildlife officers to get an idea of where these animals are being poached as well as provide insight into the networks responsible for transporting these items. By identifying at-risk areas, authorities can provide more resources for protecting those areas.
Additionally, stable isotope analysis can reveal if an animal has been taken from the wild or is being raised in captivity, away from its native habitat. This can illuminate situations of illegal captivity, as well as situations where a permit may indicate an animal was born legally in captivity but was actually poached from the wild. The latter was shown for yellow-crested cockatoos at market in Hong-Kong, a species with fewer than 2,500 remaining in the wild in a study by Andersson et al in 2021 and highlights the importance of this tool for combating the illegal wildlife trade.
The advancements in stable isotope ecology applications are an exciting prospect in the fight against illegal wildlife activity. These applications also include understanding movement and migration patterns, analyzing ecological niche over time and between species, reconstructing paleoclimate and life histories of long-extinct animals. The field of stable isotope ecology continues to expand and become more refined as our understanding of isotopic patterns and biochemical pathways grows. This is a field that brings us back to the very building blocks of life to answer complex ecological questions and save species.
Citation:
Andersson, A.A., Gibson, L., Baker, D.M., Cybulski, J.D., Wang, S., Leung, B., Chu, L.M. and Dingle, C. (2021), Stable isotope analysis as a tool to detect illegal trade in critically endangered cockatoos. Anim. Conserv., 24: 1021-1031. https://doi.org/10.1111/acv.12705
Abigail Uehling
A Unique Reference Library
I still remember the amazement I felt when I first visited and then began volunteering at the Drexel Academy of Natural Sciences in Philadelphia, close to my hometown. I took a SEPTA train downtown on a gray winter day and after a few city blocks and an elevator ride I soon found myself in a library I didn’t even know existed. Before this I had been taught how to navigate many types of reference systems – public libraries, academic libraries, online databases and archives, but it had not occurred to me that biodiversity knowledge had its very own system. During my time volunteering here, I was taught how specimens were pinned and prepared, integrated into the collection and sorted according to their changing classifications. I marveled at the sheer amount of biodiversity that was housed in a room of rolling shelves, and watched as specimens were used as a reference for a wide range of projects. I was given the task of trying to determine if some specimens needed updated names, and quickly learned that this library was far from static. Although I was just working with a single family of Lepidoptera at the time, I saw the care and meticulousness that went into ensuring these specimens were properly preserved and cataloged as accurately as possible. I also learned how important these collections are for preserving knowledge of biodiversity in our changing world.
Now as a third-year graduate student in the Invertebrate Zoology Collection at the Florida Museum, I’ve begun using these special libraries for my own research. As I have begun piecing together my dissertation dataset, I have a great appreciation for the organization and quality of this immense library. During my year as a UFBI fellow, I will be working particularly closely with the asteroid collection (Asteroidea:Echinodermata). Commonly known as sea stars, this diverse group of echinoderms is found across all ocean depths and latitudes. My research focuses on how the distribution of sea stars is influenced by ecology and connectivity across large seascapes, therefore collections are an excellent source of knowledge for determining where different genera are found. Throughout the last few years, I’ve collected occurrence coordinates, physical traits and genetic connectivity across an array of asteroid genera. I’ve learned how valuable it is to be able to walk into the asteroid section of the collection and physically examine a specimen for morphological features when I find an unexpected genetic result or need to compare different species. I’ve also learned that our knowledge of collection practices is also constantly evolving. As I’ve worked with asteroids collected from the early 1900s through the current day, I’ve seen firsthand that collection practices are always changing or being improved. Some fixation methods work great for preserving color but are poor for genetic use, while others are great for genetic use but decrease color quality. GPS quality has also certainly improved throughout the years, and some specimens are attached to more detailed locality information than others. Increased digitization of specimens has also allowed scientists to study many taxa fairly well without traveling to the collections.
I’ve also seen the many pipelines and people that are needed to keep the invertebrate collection running and understand just how integral each step is. When specimens return from the field, they must be carefully taken care to ensure they are properly preserved for scientific use and that notes on habitat and locality are accurately recorded. After specimens arrive at the museum their care is far from over. On a given day in the collections, there are curators, collections managers, students and volunteers taking care of a variety of tasks – from rehousing specimens and getting them museum ready, to sending off specimen loans and making sure those that return get put away in the proper place. There is always more to do, and usually limited space to get it done.
In the upcoming weeks, the Invertebrate Zoology collection will make an exciting move to a brand-new spacious building. Although the old collection space has served many visitors and students in its day, there are great possibilities that come with moving the specimens to a space where there will be more room and resources for all involved in this extensive library system. As the collections make their way to new shelves and higher ceilings, they are being prepped for scientists to make many more discoveries of new species and visitors to learn about the amazing diversity of marine ecosystems.
Maria Beatriz de Souza Cortez
Shifting Our Mindsets Towards Biocultural Diversity Conservation
In 2021, UFBI fellow Mahi Puri wrote a compelling and important blogpost about biodiversity loss and the ambitious targets set by the Convention on Biological Diversity (CBD) to protect it. In her article, Puri underscores how “none of the 20 targets that were set for biodiversity have been met”. Puri goes on to question the validity of setting even more ambitious targets towards biodiversity conservation for the decade of 2021-2030, especially when “bringing more public lands under protection” seems to be one of the main goals. It is very disappointing that in 10 years none of the CBD targets have been met and even more alarming that the creation of new targets seems to follow the same ambitious trajectory doomed to fail. As the blogpost suggests, creating additional protected areas is a “challenging proposition” and not as straightforward as it is made to be.
But what is so challenging about creating protected areas? Well, there are many challenges involved in this process, that range from conception to logistic. However, one of the most pressing challenges concerns the involvement of all levels of stakeholders, including local communities that rely on the land being conserved for their sustenance. Local communities are in the front lines of any type of conflict generated by and with the creation of protected areas. They are the ones that suffer with displacement, with negative direct contact with fauna, environmental disasters such as uncontrolled wildfires, etc. Yet, there are still conservation strategies and policies that insist on suggesting top-down approaches, in which local communities’ perspectives are not heard or are altogether disregarded. However, as Puri points out in her post, there are also alternative conservation strategies that propose “integrated approaches that contribute to the socio-economic development of people without displacing them”. Often, in the literature, such approaches are included in the realm of community-based conservation strategies and as suggested in the blogpost, they have shown positive results.
At this point, there is a chance that readers find themselves wondering why I started this blogpost talking about biodiversity when the title clearly states biocultural diversity. No, your eyes do not deceive you, you read it properly. This is a post about biocultural diversity conservation. Yet, it is impossible to talk about biocultural diversity conservation without considering how biodiversity conservation takes place in this world. There is no biocultural diversity conservation without biodiversity AND cultural conservation. The problem is that society tends to focus much of its efforts primarily on biodiversity conservation, by prioritizing, for instance, the creation of protected areas in which local communities are prohibited from using the land.
When thinking of conservation, generally our first thoughts (as demonstrated above) shift to the creation of protected areas as an easy solution. The idea of having areas around the globe where pristine nature thrives untouched by humans is very compelling. Yet, not realistic. First, the concept of pristine nature is debatable; how can we guarantee a certain stretch of forest, grassland or swamp has never been inhabited? According to recent research, humans have been shaping and reshaping the globe, including areas commonly believed to be untouched for over 12,000 years! This belief originates from the impacts of colonization. With the genocide of native indigenous populations, many of their cultivated lands were abandoned and further occupied by wilderness. As a consequence, after the first waves of colonization, settlers in the Americas were led to believe wilderness had always been pristine and its rich biodiversity was a result of the absence of humanity. For those of you more interested in the “myth of pristine wilderness,” I recommend the blogpost written by Claudia Geib for Sapiens; it is definitely an interesting read.
The second and perhaps more concerning issue related to the concept of thriving pristine nature is that currently many of the areas in line for being protected are the source of sustenance for local communities that live in or around them. It doesn’t seem fair, not to mention reasonable, to exclude these communities from the decision-making process and displace them from their land. Most of these communities hold extensive knowledge of their homelands (no surprise) and already employ solutions to properly care for or restore biodiversity. In the Gishwati forest, in Rwanda, a community-based conservation approach focused on the protection of the chimpanzee population has contributed to both lowering the levels of illegal cattle-grazing as well as increasing the number of chimpanzees. Across the Atlantic, communities in northwest Andean Ecuador have been actively planting trees to restore cloud forests. This effort has contributed to a more diverse forest when compared to secondary forests that are naturally regenerated. Moreover, as Puri mentioned in her blogpost, there are other types of human interference that produce much more harmful impacts, such as extensive monocultures and large-scale mining.
In Brazil, for example, the state of Minas Gerais has had to deal with the drastic consequences of two major disasters associated with large-scale mining endeavors. In 2015, a dam collapsed in the outskirts of Mariana, releasing 43.7 million cubic meters of toxic mud in the environment and killing 19 people. Fast forward to 2019 and the horror story repeats itself, now in the town of Brumadinho, but with a much more deadly outcome; 270 people were killed, and 12 million cubic meters of toxic mud were released into the environment. According to a report produced by Julia de Azevedo Oliveira while an undergraduate student at Universidade Federal Rural do Rio de Janeiro, the damages caused by the dams’ collapses were “significant, diversified and presented high magnitude”. Oliveira’s report shows a table detailing the impacts generated by the dams’ collapse on three different spheres, the physical, biological, and socioeconomic. The list is so extensive the table occupies nearly 5 pages. Among the impacts, we note deterioration of water quality, topographic alteration, worsening of species conservation (including WITHIN protected areas), probable extinction of endemic species, loss of agricultural lands, destabilization of local socioeconomic spheres, etc. And worst of all, loss of human lives and of biodiversity.
These examples demonstrate how conservation strategies focused solely on biodiversity protection, such as the creation of protected areas, are not only exclusionary but also minimally effective. They are exclusionary because they often ignore the human and cultural components necessary for biocultural diversity conservation. And they are minimally effective because they show restricted efficacy in mitigating the worst of human impact, such as the mining disasters detailed above. Moreover, community-based conservation strategies that represent a step in the direction of biocultural diversity conservation can show very positive results, as exemplified above. That is why I believe shifting our mentality towards prioritizing biocultural diversity conservation is necessary. I am not the first, nor will I be the last to advocate for this. In fact, this conversation is not even up for debate for some indigenous peoples who have always understood the connectivity between the human and more than human elements of this world. I hope we can continue to honor this knowledge as much as we value what is produced in academia so that we may have the chance to truly contemplate a future rich in diversity in all of its forms.
References:
Chancellor, R. et al. 2020. Community‐based conservation and chimpanzee research in Gishwati forest, Rwanda. American Journal of Primatology. 2021;83:e23195.
Ellis, E. et al., 2021. People have shaped most of terrestrial nature for at least 12,000 years. PNAS Vol. 118 No. 17 e2023483118.
Fletcher, M.S., Hamilton, R., Dressler, W., Palmer, L., 2021. Indigenous knowledge and the shackles of wilderness. PNAS Vol. 118 No. 40 e2022218118.
Gavin, M.C. et al. 2015. Defining biocultural approaches to conservation. Trends in Ecology & Evolution 30: 140-145.
Geib, C. 2022. How “Wilderness” Was Invented Without Indigenous Peoples. (https://www.sapiens.org/culture/pristine-wilderness-conservation/)
https://en.wikipedia.org/wiki/Brumadinho_dam_disaster
https://en.wikipedia.org/wiki/Mariana_dam_disaster
Oliveira, J.A. 2019. Impactos Socioambientais Provocados Pelo Rompimento de Barragens de Contenção de Rejeitos de Mineração no Estado de Minas Gerais. Três Rios, Rio de Janeiro.
Puri, M. 2021. Is it enough to be ambitious to reverse biodiversity loss? (https://biodiversity.research.ufl.edu/is-it-enough-to-be-ambitious-to-reverse-biodiversity-loss/)
Wilson, S.J., Rhemtulla, J.M., 2016. Acceleration and novelty: community restoration speeds recovery and transforms species composition in Andean cloud forest. Ecological Applications, 26: 203-218.
Brittany Cummings
It’s the Little Things…
Sometimes big things come in little animals. Amphipods are tiny crustaceans which comprise an abundant food source for fish. More akin to a kangaroo than a crustacean, amphipods do not have a thick carapace and use a brood pouch to protect offspring under their body (Figure 1). Species resemble shrimp with strange bodies and lifestyles. For example, neon sea fleas (Podocerus cristatus) purportedly mimic brightly colored sea slugs that are distasteful to fish; certain mast-building amphipods (Dulichia rhabdoplastis) assemble fecal rod nests off threatening sea urchin spines; skeleton shrimp (Caprellidae) adopt hyper-elongated bodies to blend into their branching habitat; and transparent floaters (Hyperiidae) ride out their entire lives on jelly hosts (Figure 2). However, these fanciful forms are difficult to find since amphipods are masters at avoiding their visual predators. As a biologist who studies amphipods, I must use both manual and molecular methods to locate and learn more about these elusive animals.
Figure 1. The general shrimp-like body form of an amphipod. Unlike other crustaceans, amphipods do not have a thick carapace and have a brood pouch to hold their offspring.
Figure 2. Examples of the extreme body diversity seen among amphipod species. Photos by Florida Museum of Natural History and Hakai Institute.
Why should we care?
Amphipod crustaceans are vital to aquatic ecosystems. There are over 10,000 amphipod species around the world which are found in all kinds of water environments: saltwater, freshwater, groundwater, and even moist land environments (Figure 3). Amphipods are decomposers that recycle nutrients and common prey items for fish consumers, including endangered Chinook salmon 1,2. As strong ecosystem interactors, amphipods are also powerful indicators of aquatic environment health 3–5. Patterns of amphipod species and distribution provide key insight into changes in marine environments.
Despite their importance to aquatic ecosystems, our knowledge of amphipod diversity and ecology remains limited. Many species live in cold and dark areas and escape detection by hiding under rocks or algae, building protective tubes, or blending into their environment. Most research has focused on the physical features of amphipod species, but body parts are fragile and vary by sex or life stage 6. Moreover, the accuracy of visual identification has been called into question by numerous discoveries of cryptic species over the past two decades 7–10. How do we improve our understanding of an animal that is hard to identify and even harder to locate?
Figure 3. The many different habitats and ecological roles of amphipods. Image from Harte Research Institute.
How do we find a pod?
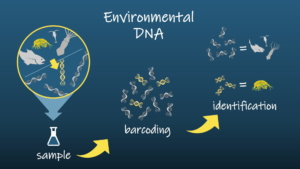
Like many active children, I often played in the “ball pit” of my favorite fast-food restaurant play area. This pit contained thousands of colorful plastic balls through which I would pretend to swim. The ocean is like a massive “ball pit” containing all kinds of tiny swimming and sedentary organisms. Instead of different colors, the “balls” are different molecular compounds. Some are inorganic molecules, such as water, and others are organic molecules, such as DNA. This DNA originates from the organisms that regularly shed cellular material into their environment. If we want to identify amphipods in this ocean “ball pit”, we have two choices: (1) collect the amphipods, or (2) collect the amphipod DNA.
Most often, I collect amphipods by hand. Manual methods are the old-school way to find and identify animals. I take clumps of algae or habitat on which the amphipod lives and then shake them off in a lab. I also use homemade “traps” which consist of common household items, such as stiff horsehair brushes and scrubby dish sponges, secured to anchors made of metal bars. These traps are placed in different ocean habitats using SCUBA and collected after amphipods take residence in its various nooks and crannies.
As a modern alternative to hand collection, I am also using environmental DNA to locate and identify amphipods. Years of genetic research on amphipods and other animals has generated a large database of DNA sequences that serve as “barcodes” 11. To identify an organism, you sample its DNA and match it against these “barcode” sequences. The principle of environmental DNA (eDNA) is to sample DNA from the environment rather than from the animal itself. Since there are countless cells in the environment, molecular tools called primers are used to pick out DNA sequences that correspond to “barcodes” of your target animal group or species. These primers are bits of complementary DNA that bind to DNA from the water sample. Once the primer binds to the DNA, it acts as a guide for enzymes in Polymerase Chain Reaction (PCR) to produce enough copies of that bound DNA for sequencing. Thus, instead of manually collecting habitat to find amphipods, I can collect water around the habitat, sequence the amphipod DNA in that sample, and match the DNA sequences to known amphipod “barcodes” for identification (Figure 4). Thanks to the guidance of Hakai Institute and supplemental funding from the NSF INTERN program this summer, I am currently testing these molecular methods for amphipod detection in coastal habitat.
Figure 4. Environmental DNA (eDNA) sampling can be used as an alternative to manual sampling of animals to identify amphipod species in complex aquatic habitats. Special designed primers attach to target animal DNA from samples of water which contain shed animal cells. The bound DNA is sequenced and matched against known “barcode” DNA sequences to identify specific animal groups or species.
Challenges
If I asked you to pick out all the red balls in a kid’s ball pit, you might think it was a hard task. But picking tiny animals or microscopic balls from an ocean? This task becomes impossibly more challenging! Both manual and molecular sampling can identify amphipod species in complex three-dimensional habitats, but both have their inherent drawbacks.
Hand collection is the simplest method for finding amphipod species but is labor intensive and invasive. If the ecology and distribution of a species is known, one can reasonably find habitat that will yield specimens. However, amphipod ecology is usually poorly known, and individuals tend to be scattered across their aquatic landscape. In this case, manual collection requires more extensive sampling of habitats to locate and identify species. This is especially problematic since many amphipods are subtidal, and sampling requires exhausting SCUBA work with long hours of substrate picking. These methods also tend to be very disruptive to the ecosystem because I must remove the animals from their home. Even with all this work, there is no guarantee that all amphipods in an area will be identified.
Environmental DNA sampling is far less invasive and provides a more complete picture of an animal community, but the results are expensive and difficult to validate. Since there is minimal environmental impact from sampling water above habitat, molecular methods allow me to acquire as many samples as needed to characterize the amphipod species in an area. This method is also far less effort after sampling since water samples can be frozen until DNA processing. However, DNA sequencing is expensive and designing primers is a frustrating trial-and-error ordeal which must be completed before sampling can begin. Moreover, the results only provide genetic information about the amphipod community which is difficult to verify without direct observation (i.e., ground truthing) of the species that are present in the sampled area.
Finding pods in the future
It is vitally important to conservation that we combine traditional and modern methods to improve our understanding of small animals that support underwater ecosystems. Amphipod crustaceans are a particularly abundant food source whose ecology has largely been ignored because they are difficult to observe and collect. As a scientist who studies amphipods, I am often limited to manual collection which is labor intensive and inefficient at finding species. However, I am developing amphipod-specific molecular methods to improve search efficiency and locate species that evade manual detection. Sampling eDNA around a habitat will allow me to extensively identify its amphipod community with minimal effort and damage; then sampling habitat in careful moderation will help verify DNA results. In this way, the strengths of either method will complement the shortcomings of its counterpart. It’s no easy task finding a needle in a haystack, but I will continue developing tools to uncover these hidden crustaceans and learn more about their important ecosystem roles.
References
(1) Orth, R. J.; van Montfrans, J. Epiphyte-Seagrass Relationships with an Emphasis on the Role of Micrograzing: A Review. Aquat Bot 1984, 18 (1–2), 43–69. https://doi.org/10.1016/0304-3770(84)90080-9.
(2) Duffy, J. E.; Hay, M. E. Strong Impacts of Grazing Amphipods on the Organization of a Benthic Community. Ecol Monogr 2000, 70 (2), 237–263. https://doi.org/10.1890/0012-9615(2000)070[0237:SIOGAO]2.0.CO;2.
(3) Thomas, J. D. Biological Monitoring and Tropical Biodiversity in Marine Environments: A Critique with Recommendations, and Comments on the Use of Amphipods as Bioindicators. J Nat Hist 1993, 27 (4), 795–806. https://doi.org/10.1080/00222939300770481.
(4) Gesteira, J. L. G.; Dauvin, J. C. Amphipods Are Good Bioindicators of the Impact of Oil Spills on Soft-Bottom Macrobenthic Communities. Mar Pollut Bull 2000, 40 (11), 1017–1027. https://doi.org/10.1016/S0025-326X(00)00046-1.
(5) Conradi, M.; López-González, P. J.; García-Gomez, C. The Amphipod Community as a Bioindicator in Algeciras Bay (Southern Iberian Peninsula) Based on a Spatio-Temporal Distribution. Marine Ecology 1997, 18 (2), 97–111. https://doi.org/10.1111/j.1439-0485.1997.tb00430.x.
(6) Chapman, J. W. Amphipoda: Gammaridea. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon; Carlton, J. T., Ed.; University of California Press: Berkeley, CA, 2007; pp 545–611.
(7) Seidel, R. A.; Lang, B. K.; Berg, D. J. Phylogeographic Analysis Reveals Multiple Cryptic Species of Amphipods (Crustacea: Amphipoda) in Chihuahuan Desert Springs. Biol Conserv 2009, 142 (10), 2303–2313. https://doi.org/10.1016/j.biocon.2009.05.003.
(8) Havermans, C.; Nagy, Z. T.; Sonet, G.; de Broyer, C.; Martin, P. DNA Barcoding Reveals New Insights into the Diversity of Antarctic Species of Orchomene Sensu Lato (Crustacea: Amphipoda: Lysianassoidea). Deep Sea Res 2 Top Stud Oceanogr 2011, 58 (1–2), 230–241. https://doi.org/10.1016/j.dsr2.2010.09.028.
(9) Beermann, J.; Westbury, M. v.; Hofreiter, M.; Hilgers, L.; Deister, F.; Neumann, H.; Raupach, M. J. Cryptic Species in a Well-Known Habitat: Applying Taxonomics to the Amphipod Genus Epimeria (Crustacea, Peracarida). Sci Rep 2018, 8 (1), 1–26. https://doi.org/10.1038/s41598-018-25225-x.
(10) Westram, A. M.; Jokela, J.; Keller, I. Hidden Biodiversity in an Ecologically Important Freshwater Amphipod: Differences in Genetic Structure between Two Cryptic Species. PLoS One 2013, 8 (8), e69576. https://doi.org/10.1371/journal.pone.0069576.
(11) National Center for Biotechnology Information (NCBI) Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/.
Adrian Barry Sosa
Microbes: The Unseen Environmental Workforce
Microbes…A collection of living organisms too tiny to be seen by the naked eye. But within those, we find the largest diversity of organisms, from Bacteria and Archaea to Fungi and algae. The collection of different genetics and metabolisms among them largely surpasses that of all organisms we can see. Sometimes, the mention of microbes induces the fear of diseases, and although some of them are our most terrible foes, they also include our most useful allies. In fact, life as we know it would have not been possible in this planet without the oxygen released by photosynthetic bacteria around 2 billion years ago. Besides, they provide direct benefits to us. For instance, an increasing body of research is strengthening the fact that gut microbiota is critical for our health. Good soil microbiomes are key to crop fertility. Microbes can also help us to produce foods as popular as cheese, bread or beer. Microorganisms are everywhere. A few decades ago, it was hard to believe that microbes could live in hot springs of nearly boiling water, in mines and caves several kilometers below the Earth’s surface and in lakes below the thick ice sheets of Antarctica. But so much of the scientific landscape has changed, that nowadays it would be surprising to find a natural environment that is completely sterile. Microbes are capable of surviving every possible challenge they face from high or low pressures, extreme temperatures, including above 100 C and below freezing, radiation, desiccation, extremely low nutrients… They were the first living beings on Earth and certainly they will be the last ones too.
Among the myriad of places where microorganisms live, we find them in the Upper Floridan Aquifer, or UFA, which is the one area that I focus my current research on. The UFA is a large underwater reservoir, where water is stored within the pores of rocks, acting like a sponge. The UFA spans underneath the whole state of Florida, reaching some parts of southern Georgia, Alabama and South Carolina. The UFA is a critical water supply for all those areas, and it is responsible to feed many surface environments as well. The majority of the hundreds of springs scattered across north-central Florida are fed by this aquifer as well. The pristine waters of the aquifer, however, present a challenge to all microorganisms living there, owing to the fact that water here is isolated from the surface. In some places, it has been estimated that water remains underground for up to 40 years. This isolation prevents high loads of nutrients, organic matter or sunlight to fuel primary productivity from reaching deeper parts of the aquifer. In order to survive this scarcity of food, microorganisms living here need to develop strategies that help them to cope with such challenges. Although much research is still needed in this area, we are starting to have evidence that food webs in the UFA are able to provide organic matter of enough quality that it is enough to overcome their low quantities. Another strategy is reduced size. The smaller, the less amount of food you need to consume to stay alive, and that certainly apply to the microbes here too. In fact, some of the groups of microbes found here include the smallest group of living creatures known thus far.
Natural microbial populations in the UFA are critical to maintain the health of the aquifer, and to make sure it can still provide water for future generations. Ensuring these populations maintain a good balance is critical for keeping a healthy environment. However, several human actions can severely impact this natural equilibrium, thus having negative consequences for the whole environment. Excessive and careless use of fertilizers releases nitrates that can make their way into the aquifer. These nitrates can fuel the activity of microbes that generate nitrous oxide, a powerful greenhouse gas. When waters contaminated with nitrates are discharged through the springs, not only this gas can be released into the atmosphere, but also excess nitrates can cause harmful algal blooms at the springs run. Wastewater seepage can drastically change the community´s composition to one where dangerous microbes, like enteric bacteria, start to be present and replace the good microorganisms. Finally, in coastal areas, water overdraft can cause saltwater intrusion that renders parts of the aquifer unsuitable for any human use.
In all, the natural microbial communities in the Floridan aquifer are doing a good job of keeping it healthy. Even if they seem tiny, the critical role of microbes in the environment cannot be neglected, as they help protecting the aquifer too. However, it is only now that we are starting to scratch the surface of this complex subterranean ecosystem, and more exciting discoveries are still awaiting us in the future.
Mitch Walters
To App or Not to App … That is the Question
About two months ago I was at my family’s cabin along the tranquil Greenbrier River in West Virginia. My dad, also a fellow birdwatcher, was one of 3 million people in the spring/summer of 2022 to download the Cornell Lab of Ornithology’s breakthrough mobile app Merlin. Using machine learning the app is able to identify several hundred North American bird species using one’s own recording. One morning we decided to put it to the test with a bird calling in a sycamore right by the river. After clicking Record, my dad pointed the iPhone’s mic toward the bird and within a couple of seconds, the app accurately identified the singing bird: Indigo Bunting. Like kids with a new toy on Christmas, we were amazed and successfully repeated the process with several other feathered friends. After our brief morning birding excursion, we tallied up 17 species and uploaded the whole list, including recordings, to another mobile app, eBird, so that any fellow eBird user could know exactly what was in our area at this time of year. It was only 7:45am, fog still clung to the Black Maples lining the shore, and I hadn’t even had my vanilla chai tea yet.
Photo of how the Merlin app operates in real time
This is just a snapshot of what today’s mobile-filled world is like for the average bird and nature enthusiast. It’s a far cry from my childhood birdwatching trips with my dad in southern California, full of dusty field guides, some random book about birdwatching in our general region of interest and printed out MapQuest directions (do you kids remember MapQuest??). It is nothing short of remarkable how far we’ve come, but is it… better?
In a lot of ways these applications are “better,” especially when it comes to scientific research. The mobile app eBird, launched in 2002, is used in almost every conceivable field of ornithology now, including spatial ecology1,2, breeding biology3,4, migration behavior5,6, population projection modeling7,8, and conservation/management9,10. Rather than only allowing researchers to collect publishable bird occurrence data, anyone with the eBird app and a passion for birds can contribute to science, one checklist upload at a time. This use of citizen science data (or “people power,” as coined in the latest issue of the Cornell Lab of Ornithology’s newsletter) is especially powerful when it comes to more inconspicuous taxa like insects. After talking to several grad students and faculty here about this particular blog post, several have claimed that the popular automated species identifier app iNaturalist has been nothing short of amazing for these smaller taxa, augmenting our knowledge of their world beyond anything we could’ve imagined.
But I believe there is a downside to the upside. As someone who is not a plant or insect person, I find myself using iNaturalist a lot these days. Yet despite experiencing the thrill of rapid field identification, I don’t find myself remembering much of anything. In a nutshell, I’m not learning my trees or my bugs.
Using iNaturalist or Merlin is kind of like using Google: we get the answer we want instantly, but an hour later we forget what we were even looking for, and we find ourselves searching for the answer again the next day! This observation troubles me, especially when it comes to teaching students. A 2017 study comparing the effectiveness of mobile identification apps versus traditional field guides found that students using traditional guides were significantly more likely to identify new species correctly than students using mobile apps11. Whether this was due to poor app design, students getting distracted with their phones, or other factors, it shows that mobile apps may not be the most effective tools for teaching field identification. It shows that there may still be room in our society for those dusty old field guides after all. Given the decline in field natural history teaching and taxonomy usage in undergraduate biology degree programs observed in several studies11,12,13, I think it’s therefore of utmost importance to continue teaching field identification the most effective and engaging way possible going forward.
That being said, I don’t think we need to abandon mobile apps entirely when it comes to teaching. In the same study cited previously, students were still optimistic about using mobile apps for learning, suggesting that these apps can still provide accessibility to relevant information outside class hours. Given the importance of citizen science data in today’s academic world, students should be encouraged to upload checklists to mobile apps like eBird so that they too can contribute to scientific research. In addition, there may also be more creative ways to use these apps that can still be beneficial to teaching, for example as shown through the iNaturalist teaching guide (https://www.inaturalist.org/pages/teacher%27s+guide). In the end, although I implore the use of traditional field identification methods when first learning one’s taxa of interest, I also agree that mobile apps can contribute greatly to science as a whole. In regards to the question asked in the title of this post: I think my answer is “to app,” but let’s just not get too carried away.
References
- Freeman BG, Strimas-Mackey M, Miller ET. 2022 Interspecific competition limits bird species’ ranges in tropical mountains. Science, 377(6604), 416-420 (DOI: 10.1126/science.abl72).
- Adde A, Casabona i Amat C, Mazerolle MJ, Darveau M, Cumming SG, O’Hara RB. 2021 Integrated modeling of waterfowl distribution in western Canada using aerial survey and citizen science (eBird) data. Ecosphere, 12(10) (https://doi.org/10.1002/ecs2.3790).
- Clark CJ. 2017 eBird records show substantial growth of the Allen’s Hummingbird (Selasphorus sasin sedentarius) population in urban Southern California. The Condor: Ornithological Applications, 119(1), 122-130 (https://doi.org/10.1650/CONDOR-16-153.1).
- Turella IZ, da Silva TL, Rumpel L, Marini MÂ. 2022 Breeding biology of swallow-tailed hummingbird (Eupetomena macroura) based on citizen science data. Ornithology Research, 1-9 (https://doi.org/10.1007/s43388-022-00098-x).
- Hedley RW. 2019 Long‐distance movements and evidence of post‐breeding elevational movements by Cassin’s Vireos. Journal of Field Ornithology, 90(4), 335-347 (https://doi.org/10.1111/jofo.12309).
- Haas EK, La Sorte FA, McCaslin HM, Belotti MC, Horton KG. 2022 The correlation between eBird community science and weather surveillance radar‐based estimates of migration phenology. Global Ecology and Biogeography (1-12) (https://doi.org/10.1111/geb.13567).
- Walker J, Taylor P. 2017 Using eBird data to model population change of migratory bird species. Avian Conservation and Ecology, 12(1) (https://doi.org/10.5751/ACE-00960-120104).
- Walker J, Taylor P. 2020 Evaluating the efficacy of eBird data for modeling historical population trajectories of North American birds and for monitoring populations of boreal and Arctic breeding species. Avian Conservation and Ecology, 15(2) (https://doi.org/10.5751/ACE-01671-150210).
- Callaghan CT, Gawlik DE. 2015 Efficacy of eBird data as an aid in conservation planning and monitoring. Journal of Field Ornithology, 86(4), 298-304 (https://doi.org/10.1111/jofo.12121).
- Stuber EF, Robinson OJ, Bjerre ER, Otto MC, Millsap BA, Zimmerman GS et al. 2022 The potential of semi-structured citizen science data as a supplement for conservation decision-making: Validating the performance of eBird against targeted avian monitoring efforts. Biological Conservation, 270 (https://doi.org/10.1016/j.biocon.2022.109556).
- Thomas RL, Fellowes MD. 2017 Effectiveness of mobile apps in teaching field-based identification skills. Journal of Biological Education, 51(2), 136-143 (https://doi.org/10.1080/00219266.2016.1177573).
- Smith D. 2004 Issues and trends in higher education biology fieldwork. Journal of Biological Education, 39(1), 6-10 (https://doi.org/10.1080/00219266.2004.9655946).
- Scott GW, Boyd M, Scott L, Colquhoun D. 2015 Barriers to biological fieldwork: What really prevents teaching out of doors? Journal of Biological Education, 49(2), 165-178 (https://doi.org/10.1080/00219266.2014.914556).
- Leather SR, Quicke DJL. 2009Where would Darwin have been without taxonomy? Journal of Biological Education, 43:2, 51-52 (https://doi.org/10.1080/00219266.2009.9656151).
Natalia Uribe-Castaneda
Socio-ecological Theoretical Frameworks to Assess Community Engagement Strategies in Support of Coral Reef Restoration
I am Natalia Uribe-Castañeda, a Colombian Fulbright Scholarship recipient with a background in marine management and experience working with communities. I began the SNRE Interdisciplinary Ecology Ph.D. program at the University of Florida in 2020. Through this program, I have strengthened my understanding of how other sciences such as Public Policy, Economics, Sociology and Communication can contribute to the success of restoration projects.
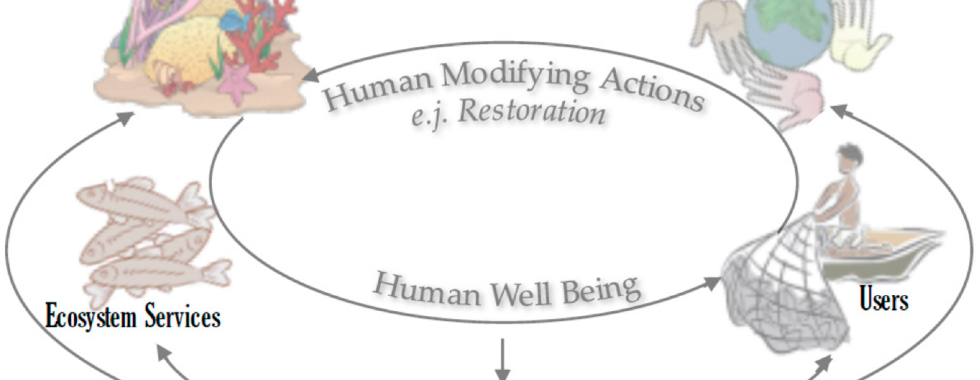
I am using socio-ecological theoretical frameworks to assess community engagement strategies in support of coral reef restoration. The socio-ecological systems approach analyzes relationships between ecosystems and society, identifying humans as a part of the system instead of separate (Ostrom 2009). Comprehension of these complex interactions is necessary when creating conservation, restoration, and management strategies (Berrouet et al. 2018).
My colleagues Alice Naewton and Martin Le Tissier (Uribe-Castañeda et al., 2018) are working in a conceptual framework to address both the socio-ecological system features of coral reefs with the implementation of restoration activity for degraded coral reefs. Such a framework can lead to better societal outcomes from restoration activities while restoring bio-physical, social and ecosystem service features of such systems. This framework can be used as a guide for managers, researchers, and decision makers to analyze the needs of coral reef restoration in a way that addresses both socio-economic and ecological objectives to analyze, design, implement and monitor reef restoration programs.
Literature
Berrouet, L.M., Machado, J., and Villegas-Palacio, C. 2018. Vulnerability of socio—Ecological systems: A conceptual Framework. Ecol. Indic. 84, 632-647.
Ostrom, E. 2009. A general framework for analyzing sustainability of social-ecological systems. Science. 325(5939), 419-422.
Uribe-Castañeda, N., Newton, A., and Le Tissier, M. 2018. Coral reef socio-ecological systems analysis & restoration. Sustainability. 10(12), 4490. https://doi.org/10.3390/su10124490
Maria Beatriz de Souza Cortez
Global Biodiversity Hotspot: Serra do Cipó National Park
During my last year of college, after returning from my 6-month internship at the University of Florida, I took a very special ecology class. This class offered me the unique opportunity to visit for the first time a place called Parque Nacional da Serra do Cipó, a protected area located in the southeast of Brazil (Serra do Cipó National Park – visit this link to learn more about the park). The park protects vegetation types occurring in the Brazilian phytogeographic region called Cerrado, listed as one of the 35 global biodiversity hotspots. Harboring over 7,000 species, the Cerrado is home to an incredible biodiversity and to high levels of endemism.
Before my visit to Serra do Cipó, I had been to other Cerrado regions in Brazil, but I had never had the chance to explore one of its most special vegetation types, the campos rupestres. The core areas of distribution for the campos rupestres occur in the Cerrado and Caatinga, but this vegetation type is distributed across all six phytogeographic regions in Brazil (Amazônia, Caatinga, Cerrado, Mata Atlântica, Pampas and Pantanal). The campos rupestres are characterized by high altitude grasslands and rocky outcrops and are prone to recurring fires. The campos rupestres can also be broadly defined as an ecoregion that includes more than the typical grasslands and rocky outcrops, like gallery and relictual forests. My ecology class offered me the opportunity to really understand what the campos rupestres were all about and to see if all the hype around it was real.
After a 10-hour bus ride we arrived late at night at Serra do Cipó and I was not able to get even a glimpse of the promised scenery. On the following day we took an exploratory trip and my first impression when I saw myself immersed in the campos rupestres was of complete awe. In my humble opinion, the campos rupestres have not earned nearly enough hype. I could string together a list of adjectives to describe this vegetation type, but there is one word that universally captures its essence. The campos rupestres are mesmerizing. It is as simple as that. To prove my point, I have added to this blog post some of the photos I took during the trip with my ecology class. It was a tough decision to come up with only a few photos, but we do not have space for over 400 photos in this blog post. Of course, these images may not elicit the strong emotions I felt when visiting the campos rupestres, but I still think they do a beautiful job in showing how unique it is.
The plant diversity in this vegetation type is remarkable; over 6,000 plant species occur in the campos rupestres and 30% of the angiosperm (= flowering plants) diversity is endemic. We spent about a week exploring and conducting open lab experiments for our final class projects. For one of them, my group and I explored the relationship between small insects and a bromeliad species. For the other project, we investigated how individuals of Drosera spp. vary in terms of leaf color according to light exposure. The genus Drosera comprises some incredibly cool carnivorous plants, with over 20 species occurring in the campos rupestres!
We also spent time getting to know the culture around Serra do Cipó. We had amazing meals prepared by the local community and I had a chance to try a few new dishes. One of them, canjiquinha, consists of roughly ground corn stewed with pork and chicken. It was simply delicious! One night, we also partook in cultural events involving dancing and we got to socialize amongst ourselves and with the community. This trip was a learning experience in so many ways and it taught us how to celebrate the beautiful biocultural diversity in Brazil. I am forever thankful to UNICAMP (State University of Campinas), to the professor that led that class, Dr. João Vasconcellos Neto, and to my colleagues that shared that experience with me.
During one of our last days, we went on an excursion to a very special place where a special endemic species occurs. Endemics are species that typically occur within a very geographic range. If I had to choose, I would say this is probably one of the fondest memories I have of this entire trip. The species we wanted to see was Vellozia gigantea; the genus Vellozia is popularly known as canela-de-ema, or “greater-rhea’s shin”. Plants in this genus received this name because their stems resemble the shins of greater rheas, flightless birds in South America occurring in open vegetation like Cerrado. We were also interested in seeing Cattleya wittigiana, this is an orchis species that grows on the stems of V. gigantea.
I remember I was walking following the trail paying close attention to the ground, since the campos rupestres are rocky grasslands with very uneven terrain. I noticed people were slowing down, so I looked up and once more I was in awe. Well, this time I was actually blown away. I felt as if I had just entered a Jurassic landscape. V. gigantea, as the name suggests, are the tallest Vellozia species reaching up to 6 meters in height! This is incredible because plants in this genus are usually herbaceous. They were everywhere and looked amazing. We started to walk around carefully trying to locate the orchid and in no time, we spotted them! They easily stood out amongst the brownish stems of V. gigantea, because the flower blossoms are of a bright red color. I remember I was so impressed that I did not really want to leave this place and so I kept looking at my surroundings trying to imprint it in my memory.
I imagine if you read up until the end of this blogpost you might be thinking I am probably not the most unbiased source when it comes to the campos rupestres given how much I love this vegetation type. That is likely true, but I hope this serves as an invitation for you to get to know the campos rupestres better and to experience it for yourself. I cannot guarantee you will fall in love with it, but I can assure you it will make a long-lasting impression on you.
Thank you to the following people for assistance with plant identification: Ana Flávia Versiane, Bárbara Leal, Cleber Chaves, Juliana Amaral, Pedro Bergamo, and Rebeca Politano Romanini.
References:
Klink CA, Machado RB. 2005. Conservation of the Brazilian Cerrado. Conservation Biology, 19 : 707–713.
Giulietti AM, Menezes NL, Pirani JR, Meguro M, Wanderley MGL. 1987. Flora da Serra do Cipó, Minas Gerais: caracterização e lista de espécies. Boletim de Botânica da Universidade de São Paulo 9 : 1–152.
Fernandes GW. 2016. The megadiverse rupestrian grassland. In: Fernandes GW ed. Ecology and Conservation of Mountaintop Grasslands in Brazil, Springer International Publishing, pp. 3–14.
Morellato LPC, Silveira FAO. 2018. Plant life in campo rupestre: New lessons from an ancient biodiversity hotspot. Flora 238 : 1–10.
Brittany Cummings
The Hunt for Marine Invertebrates
I’m on a hunt to find every kind of animal in a forest. Birds, mammals, insects, and everything in between. I look on plants, turn over rocks, and dig into the soil. I search every nook and cranny, and still miss more than a few. So, I recruit help – more people looking means more animals found.
All of the preceding is true except one key element: I’m not looking for forest animals. I’m underwater looking for marine invertebrates. This is a marine biodiversity survey.
Why marine invertebrates?
Marine invertebrates may be spineless, but they are the backbone of ocean ecosystems. In fact, invertebrate animals comprise most animal diversity on earth (Figure 1). They serve as abundant prey for fish, decomposers of dead material, and significant consumers throughout the marine food web.
Despite their importance, it’s estimated that only 11% of marine invertebrates have been described. This knowledge gap is partly because the animals themselves can be hard to find. While some macroinvertebrate species are large (hello, giant squid!), many are only millimeters long and live in dark places. Invertebrate species often escape detection by hiding, burrowing, building protective tubes, or blending into their environment. Add on the logistical problem of finding something in deep or cold water and it’s no wonder we know so little about these animals.
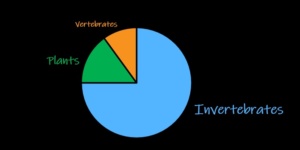
Figure 1. Invertebrates comprise most animal diversity on earth. Many of these invertebrates live in the ocean.
How do I find a marine invertebrate?
I don my wetsuit and dive underwater. Most often, I collect invertebrates by hand (Figure 2). I grab clumps of habitat that little critters live on and then shake them off in a lab. I look on algae, turn over rocks, and dig into sand. Even with all this work, I’ll still miss more than a few – there are just too many animals and too many hiding places.

Figure 2. Finding and sorting invertebrates involves a lot of gear, a lot of patience, and a lot of work!
Instead of exhausting myself in this search, I sometimes work with other people to find animals. Most often, this means diving with one extra person, but sometimes I get to work with an entire team of specialists. The latter example is a biodiversity survey called a “bioblitz”. A marine invertebrate bioblitz consists of intensive sampling and identification of invertebrates over 2-4 weeks by multiple invertebrate experts and a field crew. My advisor, Gustav Paulay, and his ragtag team of collections managers, taxonomists, and PhD students (myself included!) regularly conduct these bioblitzes around the world.
Marine invertebrate hotspots
To maximize the number of animals found during a survey, we most often target hotspots of coastal biodiversity. Coral reefs are obvious choices because they are most easily accessible (hello, shallow warm water!) and are believed to have the highest biodiversity per unit area than any other ecosystem on earth. There are generally more species per animal type in these areas than their counterparts at higher latitudes. Since these areas are so rich with life and most threatened by anthropogenic effects, bioblitzes have primarily focused on coral reefs in the past.
However, there has been recent interest in the biodiversity of upwelling zones in temperate and cold regions. Strong winds in combination with the earth’s rotation cause cold nutrient-rich water from the deep sea to “well up”. The result is high primary production and incredibly abundant fisheries. This phenomenon is most common along the west coast of continents due to westerly winds blowing water toward the shore.
Since the biggest fisheries in the world are associated with regions of upwelling, it’s important to understand what marine life support these important resources. Our lab has conducted several bioblitzes along the west coast of North America, including British Columbia, Washington, Oregon, and California (Figure 3). These intensive surveys have uncovered hidden biodiversity in most invertebrate groups. Some invertebrate types, such as amphipod crustaceans and some molluscs, are actually more diverse in these areas than in coral reefs! Other upwelling regions around the world may be hiding similar invertebrate biodiversity secrets.
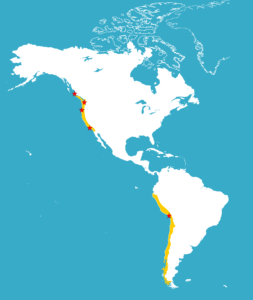
Figure 3. Sites of previous bioblitzes along the west coast of North America. Also shown is the site of the most recent archaeo-bioblitz in South America (Peru).
Go South!
The west coast of South America is also characterized by a highly productive, upwelling-driven marine system. However, half the coastline is uniquely juxtaposed against some of the driest land on earth (Figure 4). Ancient humans in this desert thrived by subsisting off of abundant ocean life. Skeletal remains of marine invertebrates and fish at archaeological sites attest to heavy reliance on marine foods for these cultures, but specific resource use is unclear because records of coastal species are incomplete.

Figure 4. Peru is home to some of the driest places on earth but is uniquely juxtaposed against some of the most productive ocean ecosystems on earth.
Thanks to funding support from the UFBI Fellowship and the Lewis & Clark Fund for Field Exploration & Research, I was able to co-lead a bioblitz along the southern coast of Peru last month (Figure 5). I worked with archaeologist Paul Pluta (UF Department of Anthropology) and Dr. Herbert Soto (Universidad Nacional de Moquegua) to document local invertebrate species in Ilo. In collaboration with local dive group Diving Ccanto, we used SCUBA diving and intertidal observations to locate animals. Individuals were carefully collected, photographed, identified, and sampled for DNA. We are using the results to build an online database of marine invertebrates to help biologists and archaeologists identify species from both modern sites and historical remains.
Just the beginning
Thanks to collaboration with the Soto lab at UNAM, I’m hoping this is just the start of biodiversity surveys along the west coast of South America. I want to expand the archaeo-zoological database by identifying more invertebrates in Ilo and south of Peru. I’m particularly excited about central and southern Chile which is at parallel latitudes to survey sites in North America. Similar to the northern hemisphere, amphipod crustaceans and shallow water molluscs in these southern locations may be more diverse than their counterparts in coral reefs and lower latitude sites. Who knows? Only the future and another ragtag bioblitz team will tell.
Abigail Uehling
Endless Invertebrate Finds in Oman’s Diverse Water
Setting the stage
This fall, during my UFBI Fellowship, I had the opportunity to participate in a BioBlitz exploring marine invertebrate biodiversity in Oman, which is nestled in one of the ocean’s most ecologically variable settings. The marine ecosystems of the Arabian Peninsula have long been recognized for their incredible diversity and high degree of endemism (Dibattista et al., 2016). These oceanic regions experience some of the most unique environmental conditions among marine regions worldwide. Salinity, temperature, and nutrient levels, among other factors, fluctuate throughout the region, setting the stage for patchy marine environments with local extremes, resulting in special faunal assemblages (Spalding et al., 2007). This November, the Paulay lab led an NSF-funded BioBlitz in the Sultanate of Oman to document the under-studied marine invertebrate biodiversity. Although we expected to find novelty in Oman’s rich waters, we were continually surprised by the marine biodiversity wonders of this beautiful country.
Where to start?
When dealing with a region as diverse as Oman, it’s tough to decide which environments to target. Prior to this fall’s BioBlitz, the Paulay lab was involved in two previous efforts to document marine diversity in Oman that focused on sampling from the northeast city of Muscat, as well as the southwestern town of Mirbat in the Dhofar governorate. Preliminary data from COI barcoding has shown that many closely related taxa exhibit molecular differences in these two regions, therefore it was decided that this round of fieldwork would focus on filling in the intermediate gaps with two locations: the island of Masirah and the city of Salalah. At both locations, we were amazed by the patchiness of habitats and the incredible amount of diversity that we encountered as a result.
Collecting strategies
Preparing for collecting organisms as general as all marine invertebrate phyla also takes a bit of strategy. Our focus groups range from less motile encrusting porifera, bryozoan, tunicates and anthozoan, to more motile organisms such as echinoderms, arthropods, molluscs and annelids. Before we took off for a day of diving, we always made sure to have a variety of essentials handy: collecting bags, jars, small vials and Ziplock bags. The most basic approach to collecting is hand-picking organisms as we spot them. Flipping rocks is often a good way to find some of the larger, more motile critters. As we placed them in our collecting jars, we had to be mindful of who gets stowed together. Some organisms are more likely to release toxins when they get agitated or go after their natural prey. Therefore, some basic understanding of these interactions can prevent unhappy outcomes. In addition to hand-collecting, we used other strategies to take mass-samples that will allow us to document more cryptic fauna. This includes brushing the surface of rocks, taking samples of encrusting barnacles, sieving sand, and plankton tows. Although these samples sometimes don’t look like much underwater, we are often surprised by just how much diversity we sort out back at the lab. In many of our dive locations, invertebrates have been sparsely sampled, therefore many of our finds were endemics or cryptic complexes that are undescribed or poorly studied.
In addition to diving, we also sampled intertidal environments, where the faunal assemblage is much different than the dive zone of 30-100 feet. Here, organisms are specifically suited to a variable environment. We planned our day around the low tides and set out with buckets to see what we can find. Just as with diving, turning over rocks is a great way to find motile critters. Some of us used pumps to reach organisms that burrow deep in the sand.

Figure 1. I carried a net and brush with me on most dives. Brushing samples have all sorts of critters such as micro-molluscs, annelids, sea-spiders and nudibranchs.

Figure 2. There are always more rocks to overturn when inter-tidaling. The zonation of habitats along the tide line becomes clear as we find different faunal assemblages.

Figure 3. The sea stars I study have predictable habits and can often be found clinging to the underside of rocks. They can easily blend in with patches of red coralline algae.
Time for a photoshoot
Once we got back from a day in the field, all animals were unpacked from collecting bags and the fun of documenting them began. First, critters got sorted into morphologically similar groups. Individuals then got assigned a field number with an initial ID. Some species are well known while others are clearly new, so some IDs are rough estimates until there is more time to carefully look at these specimens. After IDs are given, each individual got carefully photographed. This is an important step as some animals lose color or other potentially diagnostic characters when they are fixed in ethanol. Before fixation we also took a tissue sample of most individuals to ensure the preservation of high-quality tissue for DNA extraction. We generally tried to sample a few representatives of each species to account for variation between individuals.
Next steps
Collecting and documenting organisms in the field is just the beginning to our understanding of the diversity of these marine environments. As specimens get curated and studied by specialists, IDs will improve, and our understanding of Oman’s marine diversity will continue to grow. As more locations in this region are studied, we will be able to better infer the distribution of species. My dissertation research will focus on the distribution of sea stars (Asteroidea) in this region, and the modes of genetic restriction influencing speciation. The specimens collected during this BioBlitz will help me to fill in sampling gaps, and eventually infer patterns across genera. As we understand more about the patterns occurring across phyla, we will continue to build on our understanding of the mechanisms influencing biodiversity.
This BioBlitz would not have happened without the help of many people. Thank you to NSF, Emily Claereboudt, our phenomenal boat drivers and our collaborators at Sultan Qaboos University, as well as our many other helpers.
References
Dibattista, J. D., Roberts, M. B., Bouwmeester, J., Bowen, B. W., Coker, D. J., Lozano-Cortés, D. F., Howard Choat, J., Gaither, M. R., Hobbs, J. P. A., Khalil, M. T., Kochzius, M., Myers, R. F., Paulay, G., Robitzch, V. S. N., Saenz-Agudelo, P., Salas, E., Sinclair-Taylor, T. H., Toonen, R. J., Westneat, M. W., … Berumen, M. L. (2016). A review of contemporary patterns of endemism for shallow water reef fauna in the Red Sea. Journal of Biogeography, 43(3), 423–439. https://doi.org/10.1111/jbi.12649
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdaña, Z. A., Finlayson, M., Halpern, B. S., Jorge, M. A., Lombana, A., Lourie, S. A., Martin, K. D., McManus, E., Molnar, J., Recchia, C. A., & Robertson, J. (2007). Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience, 57(7), 573–583. https://doi.org/10.1641/B570707
Adrian Barry Sosa
The True Value of Science Outreach
Scientists are busy people. Why should they spend time engaging in outreach activities? That is not science, after all. To be productive, a scientist should be focusing on collecting samples, producing and analyzing data, writing papers and grants. That is the way of making an impact in the science community. But what about the other community? The broader society?
Designing and developing good, interesting outreach activities generally is viewed as a burden for a scientist. It requires being away from field, lab and computer work and the experimentation-data producing cycles that are the basis of scientific research. Moreover, it requires a set of skills that scientists may not necessarily have mastered, such as good public speaking, ability to deliver complex information in a simple, yet informative way, designing and executing learning projects and activities… just to name a few. All of that demands an extra time investment that not many busy science professionals can afford.
But outreach is impact. It is scientific impact. Although it is true that it is not the kind of impact a scientist works hard for by publishing groundbreaking results and developing innovative perspectives, it is still relevant impact. In some ways, it could even be argued that it is a more important kind of impact. Scientific outreach not only quells the thirst of human curiosity for understanding the world around us in those that do not regularly engage in scientific activities. It also serves as a way to raise awareness of global challenges, from how to better prevent spread of diseases to better manage impact of pollution on the environment. Besides, outreach activities are not just a way to inspire new generations of scientists, but are crucial to engage non-scientists in science making as well. Outreach can help to better connect with stakeholders such as politicians and companies that are the main source for research funding, ensuring they understand the value of investing in science. In addition, research sponsored by public agencies has the responsibility to show where taxpayer money has ended up. A society that demands science and is able to understand scientific output, even at a basic level, it is a stronger, more resilient society ready to understand and embrace progress.
Implicating scientists in outreach is also important to shift the paradigm on how science and scientists are perceived by the rest of society. The romantic conception of a lonely, out of the ordinary genius working in a lab having an eureka moment after another does not represent how modern science works. Modern science involves multidisciplinary teams that closely collaborate with each other, made up of normal, hard-working people. Rather than portraying scientific achievements in a lineal way, it would be instructive to show that science is built on a path with multiple branches, many of which do not lead to a meaningful destination. Even good scientists experience a good deal of unfruitful ideas and experiments before achieving a satisfactory answer. Remarking how tumultuous the path was to reach certain concepts and discoveries could better illustrate how science many times deal with uncertainties. This will help society realize that this is part of the normal science activity. That would help people understand why scientists cannot always provide a clear, definitive answer to some problems.
For all these reasons, it is important that scientists devote more time and resources to science outreach activities. In this regard, it would be very useful to include outreach specific training in science career curricula in order to make science outreach a core element of the work of a scientist, and stop it being perceived as an extra burden for the science professional. In their voyage across the uncharted oceans of knowledge, scientists cannot sail alone, ignoring the rest of the people that lives with them. They need to turn back from time to time to convince those not embarked in their trips that their paths are worth following.
Natalia Uribe-Castaneda
Science Communication
Written responses by: Natalia Uribe Castañeda
Qualifying exam questions by: Jamie Loizzo
What is social responsibility?
“Is an ethical framework in which an individual is obligated to work and cooperate with other individuals and organizations for the benefit of the community that will inherit the world that individual leaves behind” (Jensen, 2006).
What are a scientist’s social responsibilities?
Wyndham and collaborators (2021) surveyed 4,789 members of scientific and engineering organizations in the six languages of the United Nations. They found that the main scientists’ social responsibilities in order are: 1, diminish personal biases; 2, promote the interest of new generations; 3, reduce society’s risks related to their research; 4, encourage public access to their research; 5, address improper use of your research; 6, Notify professional misconduct; 7, Consider potential effects on society by their research; 8, acknowledge opposite research interpretations; 9. advocate for research that benefits society; 10. Communicate science to the broader public in an understandable way, and additionally, the scientist highlighted their responsibility of managing public research funds properly.
What is science communication?
Science Communication is “the use of appropriate skills, media, activities, and dialogue to produce one or more of the following personal responses to science: Awareness, Enjoyment, Interest, Opinion-forming, and Understanding (Burns et al., 2003). Science communication has been divided into two categories: 1 Inward-facing, communicating between scientists; and 2 Outward-facing, communicating to wider audiences (Illingworth and Grant, 2016).
What are ways scientists can communicate their research beyond typical academic channels?
There are many ways that scientists could communicate research beyond typical academic channels. Some of these ways are:
Dialogue-based science communication: scientists could promote, create, and/or facilitate conversations with the broad public. These conversations should focus on maintaining and strengthening trust and credibility while emphasizing shared goals, values, and interests related to their research (AAAS, 2018).
Social Media: Scientists could use social media to best tell stories, engage proactively with broad audiences, use scientific data to inform through measurements, and motivate and inspire others to make decisions regarding the information shared (Freberg, 2019). Research has demonstrated that social media can be used as a first step to disseminate scientific messages beyond the typical academic channels (Côté and Darling, 2018).
Social Networks: Scientists could use social networks —a web of connections and relationships people have with others— to reach audiences who do not follow journalistic media or web-based platforms, these audiences tend to be less educated and less affluent. Then the use of social networks represents a great way to communicate with underprivileged audiences (NASEM, 2017).
Blogs: Scientists could use science-related blogs to motivate audiences to learn about, follow, and discuss science. Blogs have the advantage of being able to include textual depth and provide images and video resources. Additionally, they allow audiences to communicate directly with blog authors (NASEM, 2017).
Storytelling: Scientists could use their own life stories to share their research findings with broader audiences. A well-told story can be extremely powerful —it captures attention, engages and inspires audiences, and could lead them to action (3 M State of Index, 2019).
Do you believe scientists should engage in science communication? Why or why not?
I strongly believe that scientists should engage in science communication. Because Science Communication can reinforce democratic and decision-making processes, promote credibility in scientific research, including more perspectives in their research, discuss the potential risks, benefits, and consequences of their research, encourage collaboration for identifying and solving problems, practice communication skills, and develop connections that could benefit their careers and lives (AAAS, 2018; NASEM, 2017).
And what should they do if they encounter heated comments/confrontation from public audiences about their work?
I think, it is very probable that scientist face confrontation from public audiences, especially regarding contentious topics such as genetically modified organisms, evolution, climate change, etc. People tend to confront expert information that goes against their beliefs, religions, and ethical and moral values. The AAAS (2018) investigated what are the best ways that scientists could approach possible confrontation from public audiences. They recommend that scientists: 1, think carefully about forms of dialogue that maintain and strengthen trust and credibility while being sensitive to the worldviews, values, backgrounds, and priorities of their audiences; 2, use personal warm language or frames of reference; and 3, acknowledge uncertainties or limits to scientific knowledge (AAAS, 2018).
Finally, is it a scientist’s social responsibility to advocate for their science? Why or why not? And if yes, how can they do it in a way that the public can trust them?
I consider that we, as scientists, should advocate for our science, because it is one of our responsibilities. By being privileged to have enough knowledge about certain topics, it is our duty to society to share whatever information that can benefit other beings or resources. As Wyndham and collaborators (2021) highlight communicating to the broad public the results of our research is one of our responsibilities as a scientist.
Regarding the scientist’s social responsibility to advocate for their science, an excerpt from Côté and Darling’s (2018) paper call my attention, they mentioned the important role that science plays in Policy changes. Policy changes can be carried out through the direct application of research to policy or, more often, via pressure from public awareness, which can drive or be driven by research (Côté and Darling 2018). Both ways depend upon our capacity to communicate our findings and actively engage with society.
One of the ways that scientists could advocate for their science in a way that the public can trust in us, is by partnering with trusted opinion leaders from the audiences that we are planning to reach. Another way is through collaboration with social scientists who can give us ideas about what are the beliefs, attitudes, and knowledge that influence the audiences that we are trying to reach. Finally, we could provide ways to get evaluation and feedback from our audiences to improve the quality of our outreach and boost trust (AAAS, 2018).
Literature
3 M State of Index. (2019). Scientists as storytellers guide chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://multimedia.3m.com/mws/media/1667242O/sosi-3-13-toolkit-pdf.pdf
American Association for the Advancement of Science. (2018). Scientists in Civic Life: Facilitating Dialogue-Based Communication. https://www.aaas.org/programs/dialogue-science-ethics-and-religion/engaging-scientists
Burns, T. W., O’Connor, D. J., and Stocklmayer, S. M. (2003). Science Communication: A Contemporary Definition. Public Understanding of Science, 12(2), 183–202. doi:10.1177/09636625030122004
Côté, I.M., and Darling, E.S. 2018. Scientists on Twitter: Preaching to the choir or singing from the rooftops? FACETS 3:682–694. doi:10.1139/facets-2018-0002
Freberg, K. (2019). Social media for strategic communication: Creative strategies and research-based applications. Sage Publications. 617 p.
Illingworth, S. and Grant, A. (2016). “Introduction”. Effective science communication: a practical guide to surviving as a scientist (2nd ed.). Bristol, UK; Philadelphia: IOP Publishing. pp. 1–5. doi:10.1088/978-0-7503-2520-2ch1.
Jensen, D. (2006). “Responsibility”. Endgame. Vol. II. Toronto, ON: Seven Stories Press. p. 696. ISBN 978-1583227305.
Liang, X., Su, L. Y. F., Yeo, S. K., Scheufele, D. A., Brossard, D., Xenos, M., … and Corley, E. A. (2014). Building Buzz: (Scientists) Communicating Science in New Media Environments. Journalism & mass communication quarterly, 91(4), 772-791.
National Academies of Sciences, Engineering, and Medicine. (2017). Communicating Science Effectively: A Research Agenda. Washington, DC: The National Academies Press. https://doi.org/10.17226/23674 NASEM science communication research agenda
Wyndham, J. M., Anderson, Hinkins, M.S., Ericsen, J., Olson, A., Jeske, M., Liu, R., Weeding, J., and Jaffe, R. (2021). The Social Responsibilities of Scientists and Engineers: A View from Within, American Association for the Advancement of Science.
Brittany Cummings
Coloring Outside the Lines: Part 1
Color is a vitally important trait because it’s often tied to an animal’s survival in their visual environment. Colors may function in camouflage, warning, mate choice, communication, and even thermoregulation. As visual creatures ourselves, colors are model traits for studying adaptation and evolutionary change since they’re easily identified and often amenable to experimental methods.
When we think of color in animals, we probably think of those that we see most often – the ones that live on land here with us. These animals produce their colors using pigments or structural features that interact with the available light spectrum. However, in underwater environments, we also see animals with diverse coloration. Fish and larger marine organisms such as octopus and crabs are popular study systems, but there has been relatively little exploration of coloration in smaller marine animals. My research is helping to fill this knowledge gap. I am investigating potential color adaptations in tiny aquatic crustaceans called amphipods.
The first step to understanding animal coloration is to understand light and how an animal interacts with the light in their specific habitat. In this blog post, I will discuss the perception of aquatic light environments by fish predators and how this may lead to color polymorphism in my study species.
Underwater Light
The availability and characteristics of light encountered by an organism vary greatly with their physical environment. In particular, the photic zones of aquatic environments are characterized by a significant reduction in light intensity and limited wavelength spectrum available to organisms below the surface. It is in these environments that the visual systems of fish predators have evolved to utilize local light conditions and perceive prey items.
Light environments in the photic zone are ultimately the product of matter interacting with incident light from the sun. Sunlight that penetrates the earth’s atmosphere arrives as a collection of monochromatic (i.e. single wavelength) waves through multiple planes of space. The light that hits the ocean is either reflected at the surface or refracted down the water column. Visible light traveling down the water column is strongly absorbed by water molecules or scattered by particles (and, to a lesser degree, also water molecules). Long wavelength (red) light is absorbed faster than other wavelengths, while short wavelength (blue-green) light is absorbed slowest. As more light is absorbed or scattered down the water column, light intensity decreases and the light spectrum is further narrowed to the blue-green spectrum. Thus, animals in the photic zone (0-100 m) primarily inhabit environments dominated by short wavelength (blue) light at mostly low light intensities (Figure 1).
Fish prey items often live on exposed habitat and possess reflective pigments which interact with this downwelling light to generate color. Reflective pigments selectively absorb and reflect certain light wavelengths. Pigments in crustaceans such as amphipods are most often carotenoid compounds in the exocuticle layer of their integument. Incoming light from their aquatic environment passes through the epicuticle and is selectively absorbed by these pigments. Light wavelengths which remain unabsorbed are scattered back out toward the environment where they may be received by the eyes of fish predators.
Underwater Vision
Species in the photic zone often utilize light contrast or color vision to detect prey items. Fish achieve this detection using a sophisticated visual system that we also possess as humans: the camera eye.
The camera eye generates an image by using a lens to direct incoming light onto a retina which processes light signals (Figure 2). This light will pass through a corneal lens which is refracted onto the back of the eye (retina). Since the refractive index between water and the eye is negligible (two similar media), fish possess a spherical lens to increase the refractive ability of the lens. The retina is comprised of ten layers of tissue containing neural cells that are responsible for detecting and processing light signals. The light is guided to the photoreceptive layer, which is the farthest layer to maximize photoprotection and selectively funnel light to photoreceptor cells. Camera eyes utilize two kinds of ciliary photoreceptor cells called rods and cones, which consist of a cell body and a light sensitive region facing the back of the eye. The light sensitive region consists of folded membranes containing visual opsins (i.e., opsin apoprotein attached to a chromophore). Light detection happens when light hits the chromophore and transforms the chromophore from a cis- to trans- conformation. This sends a signal down the cell body to other neural cells and eventually to the brain.

The general anatomy of a camera eye. Light in the field of view of the eye will be directed by the lens onto the retina. Photoreceptors in the retina will transfer light signals to the brain for light detection.
Different photoreceptor cells respond to different wavelengths of light which allow for color differentiation. Rod cells are responsible for detection of light and dark, and cone cells are responsible for resolution and color discrimination. Fish and other animals discriminate color and contrast based on the principle of color opponency. Rather than processing light signals in the brain separately, light signals from different photoreceptor cells are compared with one another through “color channels” to determine the color of incoming light. In this way, a fish can perceive both the image and color of a prey item against its background.
Colorful Amphipod Prey
Fish are common predators of coastal amphipod species. I study an amphipod species complex with striking color polymorphism known as Podocerus cristatus (Caprelloidea: Podoceridae). Most individuals of P. cristatus remain inconspicuous against their subtidal habitat, however, some individuals appear to resemble the bright color patterns of venomous or toxic local nudibranchs, while others fluoresce red at depths without long wavelength light. These animals belong to a group of corophioid amphipods which live on exposed habitat and exhibit extreme body modifications and symbioses which may mitigate fish predation (Figure 3). Thus, my research investigates color polymorphism in P. cristatus as an adaptation to visual predation.

Examples of corophioid amphipod diversity: (1-3) Neon sea fleas (Podocerus cristatus) resembling nudibranchs and displaying rare red fluorescence (Photos: Brittany Cummings, Matt Whalen, Merry Passage); (4) Ericthonius amphipod hiding in its protective tube (Photo: Brittany Cummings); (5) elongated skeleton shrimp (Caprellidae) camouflaging themselves against branching hydroids (Photo: Jennifer Chadwick); (6,7) whale lice (Cyamidae) clinging to a giant whale host (http://buzzmarinelife.blogspot.com/2016_08_08_archive.html); (8) Dulichia rhabdoplastis amphipods tending to their fecal rod nest which safely extends off the end of a sea urchin spine (Photo: R.L. Shimek)
Many individuals of P. cristatus possess concealing coloration which may reduce visual detection (Figure 4). Concealment confers a fitness advantage by preventing detection by another animal. Crustaceans often use concealing traits to avoid detection by common visual predators, such as fish or birds. Bottom-dwelling animals achieve concealment through plastic color traits or heritable color traits (color polymorphism) that generate background matching, disruptive patterning, or object resemblance. In the case of color polymorphism, visual predation can select for color traits that best fit the habitat associations (and, therefore, most common backgrounds) of a prey population. Cryptic coloration in P. cristatus ranges from mottled to solid pigmentation, which suggests potential color-based habitat associations for crypsis.

Example of a camouflaged Podocerus cristatus. Many individuals appear to resemble the habitat on which they are found. Photo credit: Brittany Cummings
Contrary to their camouflaged brethren, other individuals may use bright signaling to deter visual predation (Figure 5). Signaling confers fitness benefits to an animal by facilitating a specific response in another animal. Batesian mimicry is a type of signaling in which a palatable species produces a false signal that mimics the true warning signal (i.e., aposematism) of a distasteful or otherwise harmful species in order to deter a shared predator. Some bright individuals of P. cristatus display colors that resemble the warning coloration of local aeolid nudibranchs which possess chemical or nematocyst-based defenses. Thus, P. cristatus individuals that resemble local nudibranch species may generate a false warning signal to share in their model’s defensive advantage against fish predation. Other bright individuals of P. cristatus may deter visual predators by fluorescing red. If some fish predators are neophobic eaters that can detect long wavelength emissions, they may detect and avoid red fluorescent prey items that do not fit the search image of more common color types (i.e., apostatic selection). Alternatively, if predators cannot detect long wavelength emissions, red fluorescent individuals may simply generate concealment similar to cryptic coloration, or serve no functional purpose as a byproduct of chemical structure.

Examples of brightly colored Podocerus cristatus individuals. Some bright color types appear to resemble local nudibranch species such as Flabellina iodinea (A) and Orienthella trilineata (B). Others appear to fluoresce red at depths without long wavelength light (C). Photo credit (left to right, top to bottom): Allison Vitsky, Hakai Institute, Brittany Cummings, Hakai Institute, Brittany Cummings.
To Be Continued: Measuring Color
To figure out the functional role of coloration in P. cristatus, I must measure the light reflectance of these amazing color types. By comparing body color to the color of their habitat, I can better understand if camouflaged amphipods match their background, if bright amphipods match purported sea slug models or fluorescent red algae, and obtain clues as to how color functions in their overall lifestyle. But the question remains: How does one measure this seemingly intangible trait? In my next blog post, I will discuss different methods for measuring color. Stay tuned!
Adrian Barry Sosa
How the Upper Floridan Aquifer Illuminates the Search for Life Beyond Earth
For millennia, humans have wondered if they were alone in the universe. Until a century ago, it was assumed that our vicinity was populated by intelligent civilizations that mirrored or were even ahead of that on Earth. These ideas started to fade away with the advent of space exploration, which revealed that our surroundings in solar system were hostile environments apparently devoid of any life. But even if proof of extant life has not been observed at any other planetary surface beyond Earth, that should not discourage life searching efforts, especially given than life elsewhere in the solar system is more likely to be found in the subsurface. Subsurface environments provide shelter from pernicious surface conditions, like extreme temperature fluctuations and radiation, and concentrate key resources for life such as water, potential nutrients and energy sources. The discovery of cave entrances and subglacial brine lakes on Mars and ice caves within the ice crust of Europa makes them primary targets for astrobiology exploration. However, exploring these extraterrestrial subsurface environments presents many challenges. First, accessing them using remotely controlled craft has significant engineering difficulties. Second, there is the need to ensure that planetary protection protocols to avoid forward contamination are strictly followed. Third, our knowledge of subsurface environments is limited. Even here on Earth, larger efforts are still needed to fully characterize the subsurface microbiome, especially given than Earth subsurface environments host a significant part of Earth´s biomass and biodiversity.
But how a karstic aquifer like the Upper Floridan Aquifer (UFA) can help in advancing astrobiology subsurface exploration beyond Earth? One of the main challenges faced by organisms living in the subsurface are oligotrophic conditions, meaning that there are under limiting availability of organic carbon. Oligotrophic conditions are likely to occur on extraterrestrial subsurface environments as well. The UFA has a natural energy gradient with oligotrophic groundwaters and organic matter rich surface waters as endmembers, and a range of locations with different degree of mixing between the two. This gradient allows to explore microbial responses to increasing degrees of oligotrophy and how they can adapt to near-starvation conditions. For example, in oligotrophic locations of the UFA experimental results have shown that organisms living here are able to efficiently survive on high quality carbon, with relatively fast organic carbon incorporation rates. This demonstrates than even low concentrations of organic carbon can successfully support diverse microbial communities in the subsurface. In addition, the UFA, as several other karstic aquifers, is a heterogeneous environment, with taxonomical and metabolic differences between planktonic and surface-attached organisms. This heterogenicity is an important feature to consider when designing missions to explore extraterrestrial water-filled subsurface environments, since planktonic organisms may be in concentrations too low to allow any effective detection, with most of the biomass probably living attached to surfaces. The gas nitrous oxide has also been detected in the UFA. This gas is an important biosignature, so understanding how it is cycled in Earth’s subsurface is important to better characterize detection of this biosignature in other planetary bodies. Finally, the water-filled maze-like cavernous passages in the UFA are a great testbed for autonomous vehicles that can be used in extraterrestrial subsurface exploration missions, including teaching them strategies to both safely navigate through these environments and to evaluate biosignatures without direct human control.
Successful discoveries are made when scientists know what to look for and where to find it. Observations and results obtained at the UFA not only contributes to understand how life thrives within Earth’s subsurface, but their potential to host life in similar environments across the solar system. Places so close to us, like the UFA, can hold the key to discover life in places far away from Earth and make sure life, if present elsewhere, does not slip under our eyes.
Natalia Uribe-Castaneda
Community engagement in Natural Resources Management
Community Engagement
Cheuy (2018) defines community engagement as “citizens engaged in inspired action as they work and learn together on behalf of their communities to create and realize bold visions for the future.” Bishop and collaborators (2015) define community engagement as the process of involving individuals in the decision-making processes of issues that affect them. It includes activities facilitating an informed dialogue amongst participants and encouraging them to share ideas and opinions for decision-making. Mason and collaborators (2008) have defined community engagement for health promotion as ‘engaging groups of people who share geographies, interests, or identities.
Regarding the difference between Community Engagement and Community Conservation, community engagement in a general context is the set of approaches to involve communities towards an action that will benefit that community. While community conservation is a type of community engagement that focuses on engaging and empowering locals to protect their wildlife, “communities must have a reason – often an economic one – to protect their wildlife” (Wilder Institute, 2021). Community conservation can be done by generating sustainable employment opportunities, conducting education campaigns, building local capacity, and enhancing good life quality (Wilder Institute, 2021).
For the effects of my Ph.D. research, I am defining Community Engagement in the context of Natural Resources Management as: ‘approaches to involve communities in the co-management of the Natural Resources with which they cohabit’: understanding Co-management as a shared management authority among users or community groups (Ayers & Kittinger, 2014); and defining community as a group of people that share spatial, occupational, cultural, or interest-based (Ayers & Kittinger, 2014). A growing body of researchers has advocated that a better community description should include the broader social and environmental context and its networks with external actors (Stone & Nyaupane, 2014).
Community Engagement Importance
Community engagement is assumed to be essential for promoting conservation action. It is widely known that managing environmental issues requires involving the Communities (Stern, 2000). Monroe (2003) supports the importance of engaging communities in Environmental Conservation. She highlights that nowadays, people have a sense of the shortage of resources, which makes them more willing to adopt changing behaviors in favor of Nature Conservation. Ojha and collaborators (2016) assert that almost every country in the world has implemented a form of policy related to community engagement in natural resource management.
In a recent search on Google Scholar using the keywords “Communit* Engag*” AND “Conservation,” 57,300 research papers were found (Google Scholar, October 23rd, 2022). 97 % of these papers are categorized in Natural Resources Management. However, other sciences, such as Humanities, Sociology, Economy, History, Architecture, Ethics, and Health, have identified that engaging communities is vital for conservation in their respective areas (Google Scholar, October 23rd, 2022).
Community Engagement under different Socio-economic characteristics
Community engagement strategies have gained much recognition in the management of Natural Resources for their successful results (Ojha et al., 2016). Successful results are associated with the expected socio-environmental benefits, more effective environmental stewardship based on collective action (Ostrom, 1990; Agrawal & Gibson, 1999), and the potential for building human capacity to adapt to Climate Change (Ayers & Forsyth, 2009). However, critics argue that community-based strategies in natural resource management could be used as a wild card to sell programs or projects by governmental and private organizations that do not intend to develop local collective action (Ojha et al., 2016).
From African Savanah to the Great Barrier Reef, numerous research has been conducted on community engagement in natural resources management. Comprehension of the complex ecological, cultural, social, political, and economic interactions between humans and ecological systems could lead to success or failure depending on the understanding of these interactions (Uribe-Castañeda et al., 2018). The characteristics of each socio-ecological system should be understood to develop proper strategies for conserving these systems (Uribe-Castañeda et al., 2018).
For example, Cinner and collaborators (2016) compared the expected conditions of reef fish biomass versus environmental variables and socio-economic drivers. Worldwide, Conservation Organizations fostered conservation goals in low socio-economics systems by developing programs funded by interns and volunteers. They found that better conditions than expected are associated with high levels of local engagement in the management process, high dependence on coastal resources, and the presence of sociocultural governance institutions. Lundquist and Granek (2005) assert that management may be more debatable in developing countries because locals depend on natural resources for livelihood. For these reasons, strategies to engage communities in developing countries should focus on empowering locals and increasing their participation in ongoing monitoring, stewardship, and enforcement of their natural resources. Conservation success in these low socio-economic systems includes engaging communities in all stages of the restoration, management, and conservation process, good communication and clarification of expectations among stakeholder groups, the use of available science to guide the conservation and management process, understanding the importance of existence sense of place, use of traditional ecological knowledge, good governance, and funding availability (Poe et al., 2016; Turner et al., 2014; Lundquist & Granek, 2005; Dhillion et al., 2004; McGinniset al., 1999).
Failures in community base natural management in low socio-economic conditions are associated with low levels of local community involvement, government instability, lack of enforcement, lack of capacities to transfer knowledge to local community members, lack of good governance, and inaccessible, unavailable, or outdated science (Hein et al., 2019; Tuner et al., 2014; Lundquist & Granek, 2005).
On the other hand, in high socio-economic conditions, the strategies should be focused on engaging communities through citizen science and volunteer programs. The involvement of volunteers and Citizen scientists in restoration efforts has the potential to improve the stewardship of natural resources (Hein et al., 2019). Citizen science programs are designed to allow public participation to increase results toward conservation, monitoring, and research goals (Pocock et al., 2015). Some of the most common failures related to these strategies rely on the scientist’s rejection of trusting public involvement in data. However, in Coral Reef Restoration, Hesley et al. (2017) did not find any negative ecological effect from using volunteers in restoration projects; they emphasized proper training of volunteers as one of the main strategies of the programs. Additionally, Hein et al. (2019) found that volunteers and citizen science programs have, in general, low levels of local community involvement.
Literature
Agrawal, A., & Gibson, C. C. (1999). Enchantment and disenchantment: The role of community in natural resource conservation. World Development, 27(4), 629–649. http://dx.doi.org/10.1016/s0305-750x (98)00161–2.
Ayers, A. L., & Kittinger, J. N. (2014). Emergence of co-management governance for Hawai ‘i coral reef fisheries. Global Environmental Change, pp. 28, 251–262.
Ayers, J., & Forsyth, T. (2009). Community-based adaptation to climate change. Environment: Science and Policy for Sustainable Development, 51(4), 22–31.
Berrouet, L.M., Machado, J., and Villegas-Palacio, C. (2018). Vulnerability of socio—Ecological systems: A conceptual Framework. Ecol. Indic. 84, 632-647.
Bishop, K., Goller, R., Guthrie, K. (2015). Community Engagement Framework. The City of Guelph. 30 p.
Cheuy, S. (2018). Community engagement a foundational practice of community change. Community Change Festival Series.15 p.
Cinner, J. E., Huchery, C., MacNeil, M. A., Graham, N. A., McClanahan, T. R., Maina, J., & Mouillot, D. (2016). Bright spots among the world’s coral reefs. Nature, 535(7612), 416–419.
Dhillion, S.S., Aguilar-Støen, M., and Camargo-Ricalde, S.L., (2004). Integrative ecological restoration and the involvement of local communities in the Tehuacán-Cuicatlán Valley, Mexico. Environ. Conserv. 31 (1), 1–3. https://doi.org/10.1017/S0376892904001043.
Hein, M. Y., Birtles, A., Willis, B. L., Gardiner, N., Beeden, R., & Marshall, N. A. (2019). Coral restoration: Socio-ecological perspectives of benefits and limitations. Biological Conservation, pp. 229, 14–25.
Hesley, D., Burdeno, D., Drury, C., Schopmeyer, S., Lirman, D., (2017). Citizen science
Lundquist, C. J., & Granek, E. F. (2005). Strategies for successful marine conservation: integrating socio-economic, political, and scientific factors.Conserv. Biol. 19, 1771–1778. doi: 10.1111/j.1523-1739.2005.00279.x
Mason, A. R., Carr Hill, R., Myers, L. A., & Street, A. D. (2008). Establishing the economics of engaging communities in health promotion: what is desirable, what is feasible?. Critical Public Health, 18(3), 285–297.
McGinnis, M., Woolley, J., and Gamman, J., (1999). Bioregional conflict resolution: rebuilding
Monroe, M. C. (2003). Two avenues for encouraging conservation behaviors. Human Ecology Review, pp. 113–125.
O’Mara-Eves, A., Brunton. G-, and McDaid, D. Oliver, J. Kavanagh, F. Jamal, T. Matosevic, A. Harden, and J. Thomas. (2013). Data extraction and risk of bias tool for effectiveness studies. In Community engagement to reduce inequalities in health: a systematic review, meta-analysis, and economic analysis. NIHR Journals Library.
Ojha, H. R., Ford, R., Keenan, R. J., Race, D., Vega, D. C., Baral, H., & Sapkota, P. (2016). Delocalizing communities: Changing forms of community engagement in natural resources governance. World Development, 87, 274-290.
Ostrom, E. (1990). Governing the commons: the evolution of institutions for collective action/Cambridge (p. 1990). New York: Cambridge University Press.
Poe, M. R., Donatuto, J., & Satterfield, T. (2016). “Sense of Place”: human wellbeing considerations for ecological restoration in puget sound. Coast. Manag. 44, 409–426. doi: 10.1080/08920753.2016.1208037
Stern, P.C. 2000. Toward a coherent theory of environmentally significant behavior. Journal of Social Issues 56, 3, 407–424.
Stone, M. T., and Nyaupane, G. (2014). Rethinking community in community-based natural resource management. Community Development, 45(1), 17–31.
Turner, R. A., Fitzsimmons, C., Forster, J., Mahon, R., Peterson, A., & Stead, S. M. (2014). Measuring good governance for complex ecosystems: perceptions of coral reef-dependent communities in the Caribbean. Global Environmental Change, pp. 29, 105–117.
Uribe-Castañeda, N., Newton, A., and Le Tissier, M. (2018). Coral reef socio-ecological systems analysis & restoration. Sustainability. 10(12), 4490.
Wilder Institute. (2021). Community Conservation. Available at https://wilderinstitute.org/community-conservation/
Maria Beatriz de Souza Cortez
Weaving Women, Science, Creativity, and a Better Education
On Saturday, February 11, 2023, the United Nations (UN) celebrated the International Day of Women and Girls in Science. This date was created by the UN as one of the efforts to acknowledge the immense contributions of women in science, as well as to foster opportunities for young girls to get involved. In fact, to honor this important date, the Florida Museum of Natural History (FLMNH) hosted a special event on Saturday, Girls Do Science!, scheduled from 10AM to 3PM. The event was open to the public, and the goal was to promote an opportunity for girls to experience the broad and diverse science produced at FLMNH.
In the spirit of celebrating women in science, this blogpost will shine light on two very special women, Ada Lovelace and Dr. Mae C. Jemison, and their incredible contributions to science. At the same time, we will explore how these contributions were influenced by Lovelace’s and Dr. Jemison’s creativity and diverse skills sets, demonstrating how a shift in our educational system could better prepare future generations to come up with solutions to the increasingly difficult challenges our society faces.
Although Ada Lovelace passed away at the young age of 37, her scientific contributions have directly impacted the creation of computers. Recognized by most as the first computer programmer, Lovelace published the first set of algorithms to be implemented in the Analytical Engine, a machine designed by mathematician Charles Babbage. Although the machine was never built, precluding practical testing of the algorithms, Lovelace’s scientific contributions inspired Alan Turing, recognized as the father of modern computer science.

Picture 1: Left: Ada Lovelace depicted in a portrait
Right: Dr. Mae C. Jemison dressed in an astronaut suit
Mae C. Jamison was born in 1956, a little over 100 years after Lovelace’s premature death in 1852. Dr. Jamison is accredited as the first black woman to be admitted to NASA’s astronaut training program in 1987. Five years later, Dr. Jamison celebrated yet another milestone by becoming the first black woman to go to space in 1992. Before occupying a seat in the space shuttle Endeavour, Dr. Jemison graduated both as an engineer and as a physician and joined the Peace Corps for a couple of years as a general practitioner. Aboard the Endeavour, Dr. Jamison ran multiple scientific experiments over the span of eight days and was selected by NASA as one of the first Science Mission Specialists in the institution.
Apart from having impacted science in very meaningful ways, Lovelace and Dr. Jamison were also proficient in other skills sets, which have likely contributed to their professional development and success. In fact, in a recent piece published in the online periodic The Conversation (https://theconversation.com/ada-lovelaces-skills-with-language-music-and-needlepoint-contributed-to-her-pioneering-work-in-computing-193930), Lovelace’s accomplishment in computing programming is tightly linked to her skills with embroidery. In the piece, the author Dr. Corinna Schlombs states that “Lovelace began (her notes) by describing how to code instructions onto cards with punched holes, like those used for the Jacquard weaving loom, a device patented in 1804 that used punch cards to automate weaving patterns in fabric.”
Picture 2: Left: A Jacquard weaving loom, built to automate weaving of complex patterns
Right: Programming chart created by Ada Lovelace. Note the similarities between Lovelace’s work and the Jacquard weaving loom.
While Lovelace’s embroidery skills have directly impacted her scientific production, it is less clear whether Dr. Jamison’s passion for dance has impacted her professional career in the sciences. However, in a piece published in The New York Times more than 30 years ago, the author stated that “(Dr. Jamison) has long been interested in modern jazz and African dance and has choreographed and produced several shows.” Moreover, the article mentions Dr. Jamison took a poster of the prestigious Alvin Ailey American Dance Theater to her mission in space, which at least emphasizes how her passion for dance was incredibly significant to her and how she managed to sustain it while contributing to science.
But how do these creative, broadly educated, and well-rounded women make us think about improving our current educational system? Well, we do not have to leap very high to connect Lovelace’s and Dr. Jamison’s creativity and interdisciplinarity with their success. In fact, The New York Times article states that Dr. Jamison brought the Alvin Ailey poster with her as a “salute to creativity and dance”. Moreover, in The Conversation piece, Dr. Schlombs affirms that “(…) Lovelace applied knowledge from what we today think of as disparate fields in the sciences, arts and the humanities. A well-rounded thinker, she created solutions that were well ahead of her time”.

Picture 3: Left: Dr. Mae C. Jemison (right) and Dr. N. Jan Davis (left) aboard the Space Station
Right: Alvin Ailey Amercian Dance Theatre performing Revelations
It is very likely that multiple factors are involved in the equation resulting in these women’s successful contributions to science. Nonetheless, we can affirm that at least two of them, creativity and interdisciplinarity, could be much more explored in our current educational system. For example, in the university setting, the number of specialized departments is continuously growing; although this diversification leads to more targeted research, it can also lead to isolation. At the same time, undergraduate students are faced with long hours of lectures that can be inspiring, but also boring and not truly engaging. Moreover, these students must spend too many hours preparing for exams that often measure their capacity to memorize content rather than understanding it. Therefore, students could end up dedicating less time (if at all) pursuing other hobbies and activities where they are sure to exercise creativity and connectedness.
How are we preparing our students to solve increasingly difficult problems, if we are not giving them enough room to exercise their creativity both inside and outside the classroom? How are we creating opportunities to increase connections between the diverse gamut of departments and laboratories in our universities? This past Saturday I took part in the event promoted by FLMNH and was reminded of how offering the opportunity for learners to engage creatively with content is crucial to get them engaged! I saw so many different tables presenting not only specimens and scientific snippets but also an artistic and/or fun activity to attract visitors. The children were mesmerized (and so were the parents). What is stopping us from creating similar opportunities inside the classroom and throughout all educational levels?
In this blogpost, we do not pretend to have answers for all these complicated questions, but we believe that raising them represents the first step to get us moving towards change. Afterall, as Dr. Schlombs suggests, Lovelace “can be a model for all students, not just girls,” and creativity and interdisciplinarity can transform our educational system and the world.
References:
Brittany Cummings
Coloring Outside the Lines: Part 2
In my last blog post, I discussed animal coloration and exciting color polymorphism in some amphipod crustaceans. It’s obvious that an animal’s color is often vitally important because it’s tied directly to survival in their visual environments. However, it’s not as obvious to figure out how to quantify color. In this blog post, I will discuss color as a physical property that can be measured.
What is Color?
Color is a difficult concept to grasp because, well, you simply cannot “grasp” it. There are many ways to describe something you can touch, but how do you describe something that you can only see? Or, even better, that is a descriptive term in and of itself?
Things get easier if we consider color as a physical property. In its simplest definition, color is light. Light is the electromagnetic emission of energy. The entire spectrum of possible energy emissions is called the electromagnetic spectrum (Figure 1 – mention that the visible spectrum in the figure corresponds to humans). The behavior of light is dual-natured and can be thought of as “surfing” particles, or photons, that oscillate through space. Energy frequency and wavelength describe the wave nature of light, but photons are the “packets of energy” that comprise the wave.
Color results from the eye’s perception of light interactions. Light environments are ultimately the product of matter interacting with incident light from the sun. Incoming light from the sun is first scattered or absorbed by particles in the atmosphere. Sunlight that penetrates the atmosphere will strike the surface of an object and be absorbed, transmitted, reflected, scattered, refracted, diffracted, re-emitted, or some combination of the above. Color is the specific portion of the light spectrum that is reflected back from a surface and perceived by the eye.
Figure 1. The electromagnetic spectrum, showing the entire spectrum of possible energy emission (open source image). The highlighted section indicates the small portion of the electromagnetic spectrum that accounts for visible light. Image by Philip Ronan, distributed under a CC-BY-3.0 license.
Visualizing Color
Vision is an adaptation to utilize information available in a light environment. As mentioned above, the light available to visual systems is determined by the radiance spectrum illuminating the environment and the resulting light reflected by the object of focus. Human and other animal eyes have adapted to utilize energy (i.e., see) the portion of the electromagnetic spectrum which corresponds to its reflected light environment. The reflected light is a continuous radiance spectrum of different wavelengths and intensities. Instead of quantifying the entire spectrum, animals estimate the intensity of reflected light by sampling a limited number of wavebands of the spectrum. For instance, the human eye samples 3 wavebands corresponding to long, medium, and short wavelength light using red, green, and blue photoreceptor cells (i.e., cones).
Measuring color is problematic since both natural light and visual perception vary greatly with the physical environment. Aquatic habitats are characterized by a significant reduction in light intensity and limited wavelength spectrum available to organisms below the surface. Conversely, terrestrial habitats are generally characterized by higher light intensity and a broader wavelength spectrum. Organisms with visual systems are often adapted to utilize the specific intensity and wavelength properties of natural light in their surrounding environment to interpret their surroundings in a way that will enhance fitness. This means that color measurements are highly subjective unless we can standardize both the light environment and the receiver. The first part is easily solved by measuring color in one location under consistent lighting conditions. But how do we standardize the receiver?
Capturing Color
A digital SLR camera is essentially an eye with its own set of red (R), green (G), and blue (B) “photoreceptors”. These photoreceptors are called sensors. Just like our eyes, reflected light values of an object are recorded by these sensors and processed by a “brain” (in this case, computer algorithms) which compile values into an entire picture. Each brand of camera records light values in a specific predictable way. Thus, if the camera type and lens are held constant, a camera can be used to collect standardized color information.
Digital SLR cameras record light information in bits. A “bit” is a discrete unit of information in a computer (“1” or “0”), whereas a “pixel” is a discrete unit of information in an image. A pixel in a photo is composed of one R, B, and G channel that determine the color of that pixel. Thus, each digital image is essentially three different images comprised of pixels from the R, B, or G channel.
Most digital SLR cameras record images in a 12-bit pixel format to describe values from each RGB color channel. Since each bit can have one of two values (“1” or “0”), a 12-bit pixel can take on 2!*2!*2!*2!*2!*2!*2!*2!*2!*2!*2!*2! = 4096 different values for each color channel. However, since computer monitors can only typically handle 8-bit pixel format with 2!*2!*2!*2!*2!*2!*2!*2! = 256 values, the camera will convert images down to an 8-bit pixel format during post-processing. Light values recorded by sensors are displayed in an RGB histogram which shows the pixel number (i.e., intensity or brightness) recorded for light reflectance values across this 0-255 range (Figure 2).
Figure 2. An example of an RGB histogram from a digital SLR camera screen
Reflected light values from different images can be compared by transforming camera-dependent RGB values to camera-independent RGB values using a photo calibration method. By definition, light reflectance values in each RGB channel should be equal for colorless grey objects, and the relationship between different reflectance values of grey within each RGB channel should linear. However, since the radiance spectrum illuminating the object varies with the light environment and different camera settings affect the amount of light hitting the sensors, the camera RGB reflectance values need to be calibrated relative to known color values.
Camera calibration is accomplished using grey standards. These standards are typically part of “grey-scale cards” which are a series of colorless grey patches with standard reflectance values. These cards are included in each photo (or photo session, if lighting conditions remain constant through a session) and used in post-processing to generate a calibration curve. The calibration process (1) linearizes measured RGB values to known light reflectance values of each grey scale patch, and (2) equalizes the RGB channels using a correction factor, assuming equal relationships between RGB channels for grey values (Figure 3).
Figure 3. The greyscale values measured for the set of reflectance standards following the process of (1) linearization and then (2) RGB channel equalization (Stevens et al 2007)
In order to prevent loss of color data, photo calibration requires light conditions that produce appropriate reflectance values (saturation) and light distribution (exposure). Even though the RAW RGB values are unavailable until post-processing in image software, digital SLR cameras often provide an estimated RGB histogram on its screen to estimate light values for each color channel during a photoshoot. Similar to post-processing calibration of the RAW image, the RGB histogram must be calibrated using manual white balance and one colorless white or grey card (most in-camera white-balance is based on one light reflectance value, rather than a scale of values). This white balance will not affect your RAW images, but will calibrate the histogram so that you can identify saturation or exposure problems in your photos. This is important because we want to make sure none of the images have saturation clipping (light reflectance value >255 or <0) to prevent loss of light data.
Measuring Color: Applying the Photo Calibration Method
Let’s say we have carefully photographed our objects of interest with a digital SLR camera. We made sure the light environment was consistent between photos. We included a grey-scale card in each photo. We made sure the RGB histogram was evenly distributed with no loss of light data. But how do we actually go about calibrating and measuring a photograph?
One practical way of performing the photo calibration method and measuring color is to use ImageJ software (Schneider, Rasband, & Eliceiri 2012) with the Multispectral Image Calibration and Analysis (MICA) toolbox (Troscianko & Stevens 2015). MICA toolbox linearly extracts pixel data from RAW files and accounts for differences in illumination between photos by measuring reflectance values of the grey standards in your photo relative to the known values you enter into the program. The program generates a corrected image for each of the three channels (RGB) of the visible spectrum, and the user can then measure standardized RGB values for regions of interest on a photo.
The toolbox was created specifically for animal coloration and allows for all sorts of comparisons. Besides comparing average reflectance values of different objects, one can measure color pattern complexity, or even model animal visual systems through cone catch models, if the illuminant spectra and spectral camera sensitivity are known. I’m using the program to measure color in the amphipod species, Podocerus cristatus (see previous blog post). I’ll compare these measurements to reflectance values of common background types to figure out if the species is utilizing background matching or edge disruption camouflage strategies. I’m still measuring photos but am very excited to see the results!
Color is a potential science nightmare. An intangible entity that changes with the environment and with the viewer. But, thanks to modern technology, we can measure something which was previously only “in the eye of the beholder”. Certainly, color is more than meets the eye.
References
Stevens, M., Párraga, C. A., Cuthill, I. C., Partridge, J. C., & Troscianko, T. S. (2007). Using digital photography to study animal coloration. Biological Journal of the Linnean Society, 90(2), 211–237. doi:10.1111/j.1095-8312.2007.00725.x
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of Image Analysis. Nature Methods, 9(7), 671–675. doi:10.1007/978-1-84882-087-6_9
Troscianko, J., & Stevens, M. (2015). Image calibration and analysis toolbox – a free software suite for objectively measuring reflectance, colour and pattern. Methods in Ecology and Evolution, 6(11), 1320–1331. doi:10.1111/2041-210X.12439
Adrian Barry Sosa
Challenges and Outcomes of Designing an Outreach Project: My Experience as a UFBI Fellow
As the 2022-2023 academic year comes to an end, it is time to make balance on the great experience it has been for me being an UFBI fellow and summarize the challenges and outcomes that have come associated with that fellowship. The main goal of this fellowship was to translate my Ph.D. research about the microbiology of the Upper Floridan Aquifer (UFA) into an outreach project that can impact broader audiences, highlighting the importance of this environment and its tiny microbial dwellers for the heath of Florida’s groundwater and its springs, and their impacts even on regional and global scales.
Producing an outreach project came with many challenges. The most fundamental one was to come up with an outreach idea. Thankfully, in this matter I had the help of Dr. Pavlo Antonenko, who lent me a hand in the brainstorming process. Several options were put on the table: activities focused on kids at local middle schools, a series of family events at selected springs… But after a lot of thinking and consideration, I decided to do an activity in collaboration with the Florida Museum of Natural History. For this, I was very lucky to get the extremely valuable help of Janelle Pena and Alberto Torres, both from the education department at the museum. With their advice, we refined the activity that was going to be displayed: a cart in the museum. A museum cart is considered a permanent part of the museum’s exhibition, yet it is only displayed at the museum’s hall at set times during the week. In this case, the cart will be linked with the upcoming permanent exhibition “Water Shapes Florida”. These carts are run by volunteers who show museum visitors a series of dynamic activities they can interact with.
The cart contains five different activities that will allow volunteers to showcase different aspects of the UFA, its 3D structure and its associated microbes, stressing its role in the global water cycle, and its critical importance for Florida and its inhabitants. The first activity consists of a UFA model showing the aquifer’s rock layer that stores the water and the confined layer that covers the aquifer for most of its extension. A spring has been included into this model to simulate the areas of the aquifer where the confining unit is either breached or completely removed, where water can be freely discharged to the surface. Situated close to that spring a river has been built, so it can also be shown how springs are critical to provide water to surface waterways and how during wet periods river waters can intrude into some springs in what is called a reversal.
The second activity is the direct observation of microbes that inhabit the springs with the help of a microscope. Setting up this activity has represented the largest technical challenge, as the microscopy techniques required to prepare a successful permanent slide are different from those that we rutinary use for our research, so several weeks of experimentation were required to reach an acceptable outcome. This activity is accompanied by actual pictures of the slides themselves, so it is easy to guide people who may not be used to looking through a microscope to help them to visualize the sample. The third activity is also linked with the observation of the microbes and displays a series of 3D printed bacterial models to a scale of 40000x times their original size. This helps the public understand how small these organisms truly are and visualize the shape of these organisms in 3D.
The fourth activity aims to demonstrate the importance of microbial metabolism and its impact not only on the regional hydrogeochemistry, but also on a global scale. Our collaborators at the Department of Geological Sciences at UF, have detected the greenhouse gas nitrous oxide to be emitted from several UFA springs. This gas is produced as a consequence of microbial activity in the aquifer. In order to visually demonstrate the ability of microorganisms to produce gases, a balloon is attached to a bottle that contains yeast, sugar, and warm water. The yeast produces CO2, which ends up inflating the balloon. The final activity is a series of fifteen true/false cards which are written both in English and Spanish to foster inclusivity. On one side of the card, a statement about the UFA is made, and on the other, the answer is provided together with a short explanation of why it is true or false.
Being a 2022-2023 UFBI fellow has been a positive experience for me, and has greatly contributed to my Ph.D. training, providing me with real-live experience about engaging in outreach, which is a key skill for a scientist. I am grateful to the UFBI for this opportunity, which has also contributed to create a bridge between my own department (Microbiology and Cell Science) and the Florida Museum, which hopefully results into more outreach collaborations in the future.
Abigail Uehling
Star Predators
Sea stars are generally admired for their cool colors and recognizable shapes, but the roles they play in their ecosystems are not always as well appreciated. Though the impact of their presence varies depending on habitat and tropic level, many sea stars are important predators in marine ecosystems. In this post, I wanted to explore some classic examples of sea star predators, and the ways in which they shape communities across many habitats. In an age when marine environments are changing fast and biodiversity is declining, understanding the role organisms play in their habitats is increasingly important.
Why are stars good predators?
Menge (1982) attributed the predatory success of asteroids to a few main factors which include: 1) generally large bodies compared to most prey, 2) indeterminate growth, 3) unique digestive systems which are adaptable to many types of prey, 4) sensory abilities, 5) relatively quick locomotion and attachment abilities, and 5) many arms that can aid in power and attachment to prey (Menge, 1982). Additionally, many sea stars can evert their stomach out of their mouth and digest the soft tissues of their prey, which is a particularly effective way to feed on prey of different sizes and shapes.
A few case studies
There are a few sea star predators that are particularly well known in the field of ecology. In 1966, Robert Paine published “Food Web Complexity and Species Diversity”, in which he demonstrated that the predator, Pisaster ochraceus (commonly known as the ochre sea star), influences overall ecosystem functioning and diversity in the rocky intertidal of the pacific northwest. Paine experimentally excluded P. ochraceus from areas of rocky intertidal of the pacific northwest. He found that removing these top predators led to decreased diversity among the mussels, limpets, and other sessile organisms (Robert T Paine, 1966). In a healthy functioning ecosystem Piaster ochraceus would keep a single species from dominating, allowing for more diversity. Paine described this star as a “keystone species”, and this term became popularly used by ecologists to describe the important role of a species which has great effects on community structure.
A similar experiment showed that Heliaster helianthus (the sun star) plays a comparable role as a top predator along the west coast of South America. When H. helianthus was excluded from the intertidal, mussel abundance increased. However, this change in community composition was slower than that of Paine’s P. ochraceus study (Menge and Sanford 2013; Paine et al. 1985). Additional examples of sea stars predators that have been shown to heavily influence mussel populations include Asterias forbesi and Asterias vulgaris along the east coast of the US, and Stichaster australis on the west coast of New Zealand (Menge & Sanford, 2013). Although these types of experimental studies can’t account for all variation, many similar results have been found when experiments are repeated, reinforcing the concept of asteroids as “keystone” or “foundation species”. Other environmental factors, such as temperature, wave intensity and productivity, could lead to variations in population intensity, influencing predation rate and community trends (Menge & Sanford, 2013).
In contrast, the crown-of-thorns sea star, Acanthaster, causes ecosystem damage by over-grazing on corals across the Indo-West-Pacific. When populations boom, as many as 1,000 Acanthaster individuals can be found per hector; they munched coral at a rate higher than is sustainable which leads to an overall decline in coral cover (Chesher, 1969). Declining coral coverage has a predictably negative effect on the community composition and general biodiversity of these ecosystems. After coral polyps are gone, they can be quickly overgrown by algae and abandoned by fish, leading to additional cascading effects (Chesher, 1969). Although there is evidence that cycles of Acanthaster boom and bust are natural in many ecosystems, increased anthropogenic influences likely make it harder for corals to recover from effects of high Acanthaster feeding (Pratchett et al., 2017).
The detrimental effects of sea star wasting disease
The real effects of removing sea stars that are top predators are beginning to be felt in ecosystems along the west coast of North America, where the sea star wasting disease (SSWD) is affecting populations of at least 20 species from Mexico to Alaska. SSDW is caused by a sea star-associated densovirus, which causes individuals to form lesions and drop their arms, eventually resulting in death (Hewson et al., 2014). Those species affected by SSWD also include the subtidal asteroid, Pisaster helianthoides. P. helianthoides plays an important role as top predator of sea urchins, therefore its die-off could lead to a detrimental trophic cascade. If urchins populations boom without constraints, they will munch away kelp, eventually damaging entire kelp forests (Montecino-Latorre et al., 2016). Time will tell how severely these ecosystems are affected from the absence of these stars.
Smaller stars are important too!
Most of the stars mentioned above are large bodied and conspicuous in their environments, but smaller stars still play an important role lower down on the trophic system. Reading about these classic ecological examples made me think about how little we understand about the role of the many smaller sea stars. When observing smaller taxa in the field, I find many of them grazing with their stomachs everted. The genus Aquilonastra is usually found clinging under a rock or on top a rock to encrusting coralline algae, munching away. In Oman I frequently observed Fromia indica with an everted stomach over a patch of didemnid colonial tunicates. Although these taxa are much smaller and likely not as dominate in their environment, they influence community composition in ways that are not yet understood. Future experimental studies will hopefully teach us more about these lesser-known species.
References
Chesher, R. H. (1969). Destruction of Pacific Corals by the Sea Star Acanthaster planci. Science, 165(3890), 280–283.
Hewson, I., Button, J. B., Gudenkauf, B. M., Miner, B., Newton, A. L., Gaydos, J. K., Wynne, J., Groves, C. L., Hendler, G., Murray, M., Fradkin, S., Breitbart, M., Fahsbender, E., Lafferty, K. D., Kilpatrick, A. M., Miner, C. M., Raimondi, P., Lahner, L., Friedman, C. S., … Harvell, C. D. (2014). Densovirus associated with sea-star wasting disease and mass mortality. Proceedings of the National Academy of Sciences of the United States of America, 111(48), 17278–17283. https://doi.org/10.1073/pnas.1416625111
Menge, B. A. (1982). Effects of feeding on the environment: Echinoidea. In M. Jangoux & J. M. Lawrence (Eds.), Echinoderm nutrition (pp. 499–519). https://doi.org/10.1201/9781003078920-29
Menge, B. A., & Sanford, E. (2013). Ecological role of sea stars from populations to meta-ecosystems. Starfish: Biology and Ecology of the Asteroidea, 9781421410(2013), 67–80.
Montecino-Latorre, D., Eisenlord, M. E., Turner, M., Yoshioka, R., Drew Harvell, C., Pattengill-Semmens, C. V., Nichols, J. D., & Gaydos, J. K. (2016). Devastating transboundary impacts of sea starwasting disease on subtidal asteroids. PLoS ONE, 11(10), 1–21. https://doi.org/10.1371/journal.pone.0163190
Paine, R.T, & Castillo, Juan Carlos Cancino, J. (1985). Perturbation and Recovery Patterns of Starfish-Dominated Intertidal Assemblages in Chile, New Zealand, and Washington State. The American Society of Naturalists, 125(5), 679–691.
Paine, Robert T. (1966). Food Web Complexity and Species Diversity. The American Naturalist, 100(910), 65–75.
Pratchett, M. S., Caballes, C. F., Wilmes, J. C., Matthews, S., Mellin, C., Sweatman, H. P. A., Nadler, L. E., Brodie, J., Thompson, C. A., Hoey, J., Bos, A. R., Byrne, M., Messmer, V., Fortunato, S. A. V., Chen, C. C. M., Buck, A. C. E., Babcock, R. C., & Uthicke, S. (2017). Thirty years of research on crown-of-thorns starfish (1986-2016): Scientific advances and emerging opportunities. Diversity, 9(4). https://doi.org/10.3390/d9040041