2023-2024 BLOG POST
2023-2024 BLOG POST
Sikander Khare: Measuring Biodiversity in Natural Systems
Akshay Vinod Anand: Precipice…
Vanessa Luna Celino: Science Communication Efforts to Promote the Conversation on Fire Management in Peru
Ashely Hamersma: The Botany of Fire and Art of Love
Christopher Nolte: Using Genetics to Get a Glimpse into the Past
Sikander Khare: The Biodiversity-Productivity Relationship in Natural Forests: Evidence and Theory
Vanessa Luna Celino: Sorting Cards in Social-Environmental Research: Sharing Experiences About Q Methodology
Akshay Vinod Anand: Walking in the Footsteps of Historical “Birders”
Sikander Khare
Measuring Biodiversity in Natural Systems
Diversity and Rarity
“If the traveler notices a particular species and wishes to find more like it, he may turn his eyes in vain in any direction. Trees of varied forms, dimensions and colors are around him, but he rarely sees any one of them repeated. Time after time he goes towards a tree which looks like the one he seeks, but a closer examination proves it to be distinct. He may at length, perhaps, meet with a second specimen half a mile off, or may fail altogether, till on another occasion he stumbles on one by accident.” –Alfred Russel Wallace
Images taken from: https://news.mongabay.com/2015/06/how-many-tree-species-are-found-in-the-worlds-rainforests/ and https://eos.org/editors-vox/why-tropical-forests-are-important-for-our-well-being
My project for the UF Biodiversity Institute involves investigating the relationship between biodiversity and ecosystem function in US forests. In order to understand this relationship, we need to understand what diversity is in the first place. The quantification of diversity has been a controversial topic in community ecology, and numerous diversity indices abound. We may intuit that diversity is an average property of a community but what is it an average of? As illustrated evocatively in the quote above from Wallace’s (1875) description of a tropical forest, a diverse community is characterized by having many rare species: you are unlikely to find two individuals of the same species unless you sample many individuals! Patil and Taillie (1982) were the first to recognize that diversity is a measure of the average rarity of species in a community. They found that all diversity indices are a weighted average of species rarity, weighted according to their relative abundance. All diversity indices meet the following criteria:
- Rarity can be defined in many ways, but it must be a decreasing function of relative abundance i.e., the more abundant a species, the lower its rarity score.
- Increasing evenness or transferring abundance from more abundant to less abundant species should always increase diversity.
- Introducing new species should increase diversity.
By modeling the waiting time for a conspecific encounter as a stochastic process with a geometric distribution, Patil and Taillie (1982) were able to derive the equations of the three most well-known diversity indices: species richness, Shannon index, and Simpson’s index. All three indices are from a family of indices called generalized entropies because they can all be defined by a single equation with different values of an order parameter, which defines the index’s sensitivity to rare species. Unfortunately, there are many pitfalls to using these measures (Chao et al. 2014a), all of which arise from their inability to obey the replication principle (explained in greater detail below):
- Generalized entropies are measured in different units and changes in magnitude are difficult to compare or interpret.
- Apart from species richness, they do not behave in an intuitive or linear way so in highly diverse communities, mass extinctions may hardly register due to the loss of primarily rare species.
- Similarity measures based on generalized entropies do not quantify compositional similarity for cross-assemblage comparisons.
Hill numbers as unifying framework
Recently, Hill numbers have been proposed as the best choice for quantifying abundance-based diversity as well as a unifying framework for integrating species/taxonomic diversity, phylogenetic diversity, and functional trait diversity (Chao et al. 2014a). First discovered by Hill (1973) and later reintroduced in ecology by Jost (2006), Hill numbers are another a parametric family of diversity indices that can convert generalized entropy indices to effective number of species. Just as with the generalized entropy indices, Hill numbers define a rarity score for each species and average them in different ways that favor common vs. rare species, depending on the order parameter (Roswell et al. 2021):
- Species richness: is the arithmetic mean of species rarity, favoring rare species
- Hill-Shannon diversity: is the geometric mean of species rarity, favoring common and rare species equally
- Hill-Simpson diversity: is the harmonic mean of species rarity, favoring common species
Hill diversity is always expressed in terms of effective number of species, which is the same as the number of species in a completely even community with the same diversity score. As a result, regardless of how species rarity is averaged, all Hill numbers are expressed in the same units, which are readily comparable and interpretable. Moreover, Hill numbers obey the replication principle, meaning pooling assemblages with no overlapping species will result in a total diversity equal to the sum of the diversities of each assemblage. Hill number can also provide better cross-assemblage comparisons, as they can be partitioned into within- and between- group components (Chao et al. 2014a). The diversity profile of a community, given by the Hill diversity for different values of the order parameter, provides the same information as a species accumulation curve (Chao et al. 2014b), meaning it can be used to estimate “true” community diversity from sample diversity (Roswell et al. 2021). Hill numbers also can be generalized to phylogenetic and functional trait diversity (Chao et al. 2014a):
- Species/taxonomic diversity: Hill numbers represent the effective number of species/taxa. Each species/taxon is treated as equally distinct and weighted by relative abundance.
- Phylogenetic diversity: Hill numbers represent the effective number of equally distinct phylogenetic entities. Each branch segment is weighted according to branch length and relative abundance.
- Functional trait diversity: Hill numbers represent the effective number of equally distinct functional entities. Each functional entity is weighted according to functional trait distance to other functional entities and relative abundance.
Inferring process and mechanism from biodiversity-ecosystem function studies
Linking biodiversity change to ecological mechanisms has remained elusive (Godsoe et al. 2021, 2022, 2023). It has been shown that an observed change in diversity is theoretically compatible with any type of biotic interaction. Biotic interactions change absolute abundances, but diversity only depends on relative abundances. Therefore, changes in diversity often reflect the success of rare species relative to common ones (Godsoe et al. 2023). But what about in biodiversity-ecosystem function studies, where biodiversity is posited to effect change in some ecosystem variable? Diversity indices are measures of average rarity, and as Patil and Taillie (1982) showed, the rarity functions can be a model of a waiting time stochastic process. Many advances in population genetics, phylogenetics, and stochastic population dynamics have come from modeling waiting time stochastic processes. By using modern model selection methods (Ponciano and Taper 2019) to choose the Hill number order parameter that best explains the data, could we gain insight into the stochastic processes that generate the data as well as the relative contribution of common and rare species?
References
Chao, A., C.-H. Chiu, and L. Jost. 2014a. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annual Review of Ecology, Evolution, and Systematics 45:297–324.
Chao, A., N. J. Gotelli, T. C. Hsieh, E. L. Sander, K. H. Ma, R. K. Colwell, and A. M. Ellison. 2014b. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecological Monographs 84:45–67.
Godsoe, W., P. J. Bellingham, and E. Moltchanova. 2022. Disentangling Niche Theory and Beta Diversity Change. The American Naturalist 199:510–522.
Godsoe, W., K. E. Eisen, D. Stanton, and K. M. Sirianni. 2021. Selection and biodiversity change. Theoretical Ecology 14:367–379.
Godsoe, W., R. Murray, and R. Iritani. 2023. Species interactions and diversity: a unified framework using Hill numbers. Oikos 2023.
Hill, M. O. 1973. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 54:427–432.
Jost, L. 2006. Entropy and diversity. Oikos 113:363–375.
Patil, G. P., and C. Taillie. 1982. Diversity as a Concept and its Measurement. Journal of the American Statistical Association 77:548.
Ponciano, J. M., and M. L. Taper. 2019. Model Projections in Model Space: A Geometric Interpretation of the AIC Allows Estimating the Distance Between Truth and Approximating Models. Frontiers in Ecology and Evolution 7.
Roswell, M., J. Dushoff, and R. Winfree. 2021. A conceptual guide to measuring species diversity. Oikos 130:321–338.
Wallace, A. R. 1875. Tropical Nature and Other Essays. MacMillan, London.
Akshay Vinod Anand
Precipice:
A flicker on the adjacent hill, a beam of a flashlight. Humans. The distance, 10 km. But 50 feet up a rock face, with no moonlight, no water, no food and dense forest between us and the mysterious beam of hope, it was a losing battle. We watched the beam fade away into the loud darkness.
Retracing…
The Western Ghats are an ancient mountain range that runs along the western coast of the Indian subcontinent. Known for its incredible biodiversity and rain-soaked forests, these mountains are home to the charismatic Bengal Tiger, majestic Asian Elephant and a myriad other bird, insect, reptile, and plant species. I was on assignment to survey the northern regions of this beautiful mountain range for signs of large mammals to estimate prey densities for large carnivores. The northern Western Ghats are typically drier and hotter than the southern parts of the mountain range, characterized by thick scrub transitioning to moist deciduous forests. At the peak of the Indian summer, this landscape can be rather unforgiving, with temperatures exceeding 110 F.
It was early in May when I joined Mr. Sashi, the project lead, in the field. As a two-person team we were to survey close to 400 km of lowland scrub forest over the next month. This entailed walking 10 – 15 km line transects, on a daily basis, through this harsh terrain. We tried to use trails as often as possible, but being a fairly unexplored region of the Ghats, we would often end up using animal paths and dried streams to navigate through the forest. This might not have been the most ideal strategy (as we eventually found out), especially since those same paths were being walked by tigers and leopards.
This was just another day, another transect, another adventure. Or so we thought.
Sashi and I woke up rather lethargically, we had planned the day’s transect the previous night and it was going to be one of the easier ones. An approximate 8 km walk (piece of cake!). We packed our field bags and set off in our rickety old jeep. We were particularly upbeat this morning, after weeks of long and arduous surveys, this transect seemed like the perfect opportunity to enjoy the kaleidoscope of brown-green slopes that is the Ghats in summer. After a bump, a jump, and a push our jeep makes it to our starting point. We unloaded, got our bearings, and started the day’s transect.
Entering the forest is a lesson in time travel. The world around you cease to exist, the cities, the cars, the people all fade away. What is left is just you and your immediate surroundings. Time is almost nonexistent. The forest opens up and engulfs you in its calm, cacophony, and you exist as just another life form among the multitude of living energies around you. The trees lead the way, the insects laugh at you, the birds orchestrate the most beautiful symphony. Everything falls in place, like a jigsaw puzzle without its cracks.
We walk, in an almost hyperaware, meditative state. Every rustle of leaves, every twig breaking, sets off alarm bells. We play the role of a prey species, always on the lookout for danger.
The terrain is harsh, 60 º slopes, loose gravel, and a canopy 4 feet in height. A canopy of thorny shrubs. We crawl. Progress is slow. We are no longer on recognizable paths. We crawl some more. Progress is painstakingly slow. The sun is beating down and sweat drips through every pore, depleting vital fluids. The “easy transect” was proving to be anything but. We finally reach a clearing, and we take a much-needed break to plan the route forward. The GPS must be lying, it’s 3 o’clock in the afternoon and we have only done 3 km. WHAT?
No more stops, we need to motor ahead if we’re going to have any chance of making it to our destination before sundown. We rehydrate, and head north. There is approximately 4 hours of daylight and 5 km to cover. A steep, “uphill” task. We walk, the terrain is no different, thorns, gravel, sun. It’s 6 o’clock now and we are defeated. We’re down to the last liter of water, although the end is in sight. Our GPS (that sly, untrustworthy GPS) tells us that we are just 2 km from the end point. What it doesn’t show is between us and our destination, are two steep ridges, the latter with a 150-foot rock face.
We push, one ridge down, 7 o’clock, light is fading.
The thought occurs to us that we might not make it. We motor up the last ridge, as we reach the top we are confronted by a thicket of Karvy (a small shrub that grows in dense thickets, making it nearly impossible to move through). I look at Sashi, “What now?” We push and shove our way through the Karvy, as it stands there smirking at us like a headmaster whose just caught you cheating on a test. We finally break through the Karvy. The sun has set, we have about 20 minutes of ambient light and in front of us is a 150-foot, sheer rock face.
By now we are running solely on adrenalin. We decided to climb, I led the way. No way.
Rim rocked: “The state of being stuck on a rock face, with no possibility of going up or down.”
The light had faded completely, and so had the adrenaline. We were rim rocked. I spotted a ledge nearby, about 3 feet wide and 10 feet in length. We had no other option than to camp there for the night.
We settled in, coming to terms with the situation we had gotten ourselves into. As the light faded to pitch black. It started. The forest yawned, stretched, shook itself and woke up. The night had begun, and the forest was alive.
We sat in silence as the cacophony of crickets enveloped us. A concert of chirps, clicks and buzzes intertwined into a divine symphony, of which Bessie, Bach or Beethoven would be proud. The music spread like waves, rhythmic, constant, calm.
We just sat there in awe, listening, thinking, being. There was a soft rustle of the undergrowth to our right. There had been many such sounds over the last few hours, but somehow this felt different. What followed was a sound I will never forget. It was soft at first but grew in amplitude until it resonated in our bones. The low, thunderous, rumbling of a panting leopard. It was a guttural sound, propagating from every cell of the animal’s body. The panting got louder and louder; it was moving towards us.
I could feel my heart racing, until it was thudding against my ribcage. The adrenaline, that I thought was completely depleted, started coursing through my veins again. We looked at each other, frozen. You never really understand what it means to fear for your life, until you are face to face with an animal of this caliber. Leopards are expert climbers, with an acute sense of smell and hearing. The odds were stacked against us. The panting neared, till it was just below us. Then there was sniffing. We listened as the animal circled below us. Then, silence.
The next hour was the longest of my life. Every second was an eternity.
Mud and rocks come cascading down the rock face and we hear something walking above us. The leopard. My heart is in my mouth. Silence.
Panting and sniffing below us, an hour had passed. A shower of mud and rocks an hour later. This animal was having fun, “scare the pesky little humans” was the name of the game. Although, I presume it was just investigating the new smell in its territory.
The leopard eventually moved on, but not before shaving off a good 5 years from both of our lives.

A leopard pugmark, photographed the morning after our encounter, about 50 feet from where we spent the night.
We didn’t sleep a wink that night. We just drifted with the multitude of life around us, somehow incorporated into this system, like a cell performing its function to keep the entire body alive.
Sometime during the night, we saw humans on the adjacent hill. Probably the rest of our team worriedly searching for us along motorable roads. Unfortunately, this searching strategy proved to be rather ineffective.
Before we knew it the eastern sky stared to fade into existence, sunrise. As the sun emerged, and washed the forest with light, so did a pair of Langurs. They were sitting on a branch opposite us, at eye level, looking at us quizzically. Had they been there the whole night, watching us in amusement like a late-night sitcom. The expression on their faces said yes.
We decided to climb down and find a way around the rock face. We were drained, the night had taken its toll on us. I turned around and looked up one last time. To that little precipice where I lived a lifetime, in 8 hours.
gy Information. https://www.ncbi.nlm.nih.gov/.
Vanessa Luna Celino
Science Communication Efforts to Promote the Conversation on Fire Management in Peru
My doctoral research and practitioner work focuses on community-based fire management and stakeholders’ perceptions of fire governance in Peru, my home country. My research advances our understanding of the human-fire relationship by bringing theoretical and methodological approaches from multiple disciplines (ecology, geography, political sciences, and anthropology). Research and practitioner work on these issues, which are unique for the country, expects to contribute to policy making through crucial scholarship and collaborative activities with local partners. In my last in the Ph.D. program, I have been working on results dissemination by elaborating peer-reviewed scientific papers and science communication efforts. The later included the filming of a mini-documentary about my research (see below), and participatory workshops and symposia in November 2023. These efforts were possible thanks to the SNRE’s Robin E. Nadeau Ecology Graduate Research Award.
A mini documentary about my research
Research context and motivation:
Fire is an essential tool in tropical subsistence agriculture and is a traditional, intergenerational, and cash-free way of releasing nutrients, controlling pests and weeds, and preparing new farmland. However, agricultural burns and other ignition sources have increasingly affected fire regimes -frequency, intensity, severity, and seasonality- in most tropical ecosystems. Some agricultural burns result in accidental or escaped fires, where secondary outbreaks unintentionally affect surrounding areas, especially when strong winds are combined with droughts. Concurrent changes in global and regional climate also accentuate the increase in fire frequency.
Globally, societies have responded to escaped fire threats with some degree of fire suppression and prevention strategies. More recently, there has been a call for new practices and paradigms on fire management: a call that incorporates traditional ecological knowledge from indigenous and local peoples, avoids the overcriminalization of fire-related practices, and promotes bottom-up fire management. Fire governance involves decision-makers engaging with key actors to determine fire’s social and ecological role and how to manage fire. Understanding key actors’ perceptions is the first step in fire governance and can be applied to understand policy context, facilitate communication and transparent decision-making, and manage conflicts.
Specifically, the Peruvian Andes has a long history of anthropogenic fires, which have shaped the natural landscape over millennia. Here, too, fire is an important agricultural tool in local communities and the most affordable way to open farmlands and control undesirable vegetation and pests. However, as in many other tropical countries, agricultural burns are prohibited and are a significant problem when not adequately controlled. There are also interesting examples of local communities self-organizing and adapting their fire-related practices to get the most benefit from their burns and reduce wildfire risk. Yet, bottom-up fire management initiatives require the recognition of multiple actors involved in decision-making on fire related policies.
Science communication for environmental policy change:
For the dissemination and validation phase of my doctoral project, I organized and facilitated two workshops and two symposia. In collaboration with a local nonprofit organization, CEDES Apurimac, the organization of two workshops on ‘Locally-based fire management in the Peruvian Andes,’ targeted local stakeholders representing the regional and local government, nonprofit organizations, volunteer firefighters, local communities, and researchers, many of whom participated in previous phases of my doctoral research. One workshop was held in Kiuñalla campesino community and another in Abancay city.
Having my doctoral results as a starting point of discussion, participants discussed what actions were needed to promote effective fire governance in the Peruvian Andes. Overall, these workshops promoted the reflection on various current and potential fire management strategies, such as community brigades, law enforcement, fire awareness campaigns, safe agricultural burns, and landscape management.
The two symposia targeted ~250 undergraduate students from local universities in the regions where my research was conducted and aimed to promote the dialogue on fire management in Peru. In Abancay city, the symposium ‘Prevention and control of wildfires from rural communities’ brought together representatives of government, non-profit organizations, and campesino communities to discuss farmers efforts in community-based fire management. In Cusco city, the symposium ‘Research on wildfires and local prevention efforts,’ brought together fire researchers and regional practitioners in the aim to promote more wildfire research and innovative fire-related actions in Peru. Both symposia were free of charge for any attendee.
Final reflections:
Wildfires are an issue increasingly affecting every corner of the world. The focus of my research and practitioner efforts in fire governance and management is intentionally geared towards aiding decision-making through an integrated and adaptive approach to fire management. This stands in contrast to the prevailing emphasis on wildfire suppression and prevention strategies currently implemented in Peru. My main goal as a doctoral student has been to promote the bridge between research and action through science communication and spaces of social learning on effective fire-related practices and policies. This is why the organization of workshops and symposia were extremely relevant to the success of my doctoral project. Following the completion of my doctorate, I intend to persist in collaborating with rural communities, whether in academia, government, or within a non-profit organization. My goal is to devise solutions that enhance human well-being and contribute to the preservation of natural areas in tropical regions.
Hyperlinks:
Mini documentary: https://www.youtube.com/watch?v=a8JfpR0qxg0
Symposium in Abancay: https://sites.google.com/view/conferenciafuegocusco/simposio-abancay?authuser=0
Symposium in Cusco: https://sites.google.com/view/conferenciafuegocusco/inicio?authuser=0
Ashley Hamersma
The Botany of Fire and Art of Love
It is mid-afternoon. Hot – ninety degrees, though I won’t know that until I read weather reports later. More humid than usual. Squatting in the middle of a charred gum forest, I’m willing my laptop to go faster, the thin green progress bar matching the moss around me in growth speed. I glance up – thin clouds are beginning to blot out the sun. I look anywhere but the computer screen, fiddling with the latch on the game camera from which I’m copying the SD card, my heart pounding. On the crest of the hill and down, charcoal gum spires and precocious saplings are challenged by the verdant green of flourishing Darwinia, this species found only here in this two-acre plot and a few other places.

Flowers inside the colorful bracts of a Darwinia. Note the deep magenta petals, white – pink bracts, and magenta style with brush-like tip.
Quartzite-littered soil, rocky and thin, is alive with humps of moss and tiny Stylidium and Drosera. The place is thriving after the burn, really, but the spindly young trees are no match for wind, and they writhe with the announcement of a mountain storm. What seems like a hundred tons of purple-black sky, loud, angry, and churning down the side of the Stirling Range, is headed directly for me. While I’ve covered my bases for safety, I am still terrified of thunderstorms. I’m vaguely aware that I do not have my waterproof pack cover and I am running out of time before I am flattened by a wall of water, and as I watch lightning arc over the cloudfront, I realize it’s time to go. Now.

The storm, view from the farmhouse driveway
In an instant, the sun is gone. The temperature drops what seems like 30 degrees in 10 seconds. My laptop dings, and with electric focus and shaking hands I jam the SD card back into the camera, lash it to the trunk of a blackened Xanthorrhoea, test the angle, tighten my backpack straps, glance at my GPS, and start running.

The storm in my rearview as I made my escape.
________________
Back in the farmhouse, my field clothes are streaked with ash. The power is out, and the farmer’s wife is making sausages by flashlight when her husband appears through the driving rain, pausing in the house only long enough to grab boots and a coat. He hollers something I can’t catch over his shoulder, and the next thing we see is his taillights as his old truck peels down the already-muddy lane. It dawns on me with a shiver what he’s said.
“Three ignitions.”
Three fires, started by the dry lightning ahead of the torrential rain. One in the Stirlings, two nearby – the farmer is part of a local volunteer firefighting group. Out here, conflagrations can rapidly swell to monstrous, catastrophic size, far before any other help comes and quickly gaining enough power to rip through entire wheat-based livelihoods, neighborhoods, and conserved areas. It’s just before harvest. The farmer’s wife plunks a dusty, well-worn satellite radio on the table. She hands me a cup of tea.
“Hot drink?”
The soaking rain and harrowing afternoon have left me chilled, and I nod, grateful. We sip our teas in silence. The only thing to do is nervously flick through the radio channels and eat our sausages, hoping the combination of quick-thinking and rain minimizes the damage.
This time, it does, and forty-five minutes later, soaked through and ravenous, the farmer and several hands trudge back into the kitchen. We warm up dinner. The rain has extinguished the fires — part of a wheat field obliterated prior, but no other losses save some time and energy. On my three-hour drive back to Perth, I am thinking about fire, and love.
The farmer’s bush block up on the ridge would likely have been safe from these flames had they grown. Out of care for his population of Darwinia and the other rare plants his land supports, a few years back he had set the fire that blackened the gums and Xanthorrhoea and left my clothes marked with soot. After centuries of denigrating and misunderstanding First Nations fire practices and attempting to subdue the willful Western Australian countryside, settler thought is now slowly changing. This is no doubt spurred on by complete helplessness in the face of catastrophic bushfire, magnified by a changing climate and unsuitable management. Indigenous peoples comprise only 5% of the world population but protect an estimated 85% of world biodiversity through land stewardship. Working together with the bush by way of Indigenous fire stewardship, not the land’s subjugation, is essential to biodiversity conservation and human existence going forward (Hoffman et al., 2021).
To love a place, you need to recognize when what you are doing is not working. You cannot turn away from the reality of increasing destruction and bleak conservation futures just because you have done something a certain way for so long. Every single person I met in WA holds the land so close to their hearts as for it to be an extension of their own person. They are proud of their subsistence, often separated from water and electricity infrastructure, and adore their incredible endemic plant and animal diversity. They watch with bated breath and twinkling eyes as we sift through game camera footage to identify pollinators and ask questions about our yards of plastic tubing and dozens of baggies and ties as we sample volatile chemicals from inflorescences. They hover like concerned parents as we take clippings and mount cameras – pointing out here another good plant to sample, here a rare lizard, here some seeds for our library. Granted, arguments are common, and tensions are high when discussing fire regimes, and First Nations firekeepers are yet underrepresented in such discussions, but almost everyone agrees: burning is necessary. Good fire reduces catastrophe and preserves the future. This is love.
_______________
I’m back in the states now, my time as a field technician having come to a close for the year. Everywhere, I am reminded of Western Australia’s biodiversity and the people who love it. Here in North Central Florida, we have around 5 species of Drosera and around a dozen Urtricularia to WA’s hundreds and dozens, respectively, but I still dutifully seek them out, hidden as they are nestled in their bogs and flatwoods. She-oak (Allocasuarina) has been replaced with live oak, and memories blur as I walk under their dappled sunlight. A week and a half ago, my roommate went out for a run and ended up diverted, as a prescribed burn crept through the conservation area that abuts our backyard. Palmettos, bluegrass, rushes, and sapling oaks fell to ember, golden Gelsemium corollas raining down from the vines in the days after as their rootstock withered. Florida’s flatwoods are no stranger to fire, with burn cycles as short as 1-2 years in open scrub and savannah habitats (Rother et al., 2020). These environments burn readily, at low intensities, and recover rapidly, and as such this burn stayed low, slow, and gentle, filling our yard with smoke for a day and then leaving fertilizing ash and undergrowth openings for the native seedbank to fill.

The previously burnt area where we were to set up wildlife cameras.
Five days later, fiddleheads were already pushing their way through the debris. Yesterday, brilliant green shoots poked towards the sky through the bluegrass hummocks. A few bigger pines are still smoldering even after our recent rain. For the past few days, I’ve gone out to sit by them and watch the flames consume, returning the timber gently and unceasingly to nourishing dust. Warm, reciprocal, tenacious: we could learn from fire how to love our world.

New fern growth in the flat woods burn behind the place I live in the outskirts of Gainesville with embers still glowing in the background.

New fern growth in the flat woods burn behind the place I live in the outskirts of Gainesville.
References
Hoffman, K. M., Davis, E. L., Wickham, S. B., Schang, K., Johnson, A., Larking, T., Lauriault, P. N., Quynh Le, N., Swerdfager, E., & Trant, A. J. (2021). Conservation of Earth’s biodiversity is embedded in indigenous fire stewardship. Proceedings of the National Academy of Sciences, 118(32). https://doi.org/10.1073/pnas.2105073118
Howard, T., Burrows, N., Smith, T., Daniel, G., & McCaw, L. (2020). A framework for prioritising prescribed burning on public land in Western Australia. International Journal of Wildland Fire, 29(5), 314. https://doi.org/10.1071/wf19029
Prober, S. M., Yuen, E., O’Connor, M. H., & Schultz, L. (2016). Ngadju Kala: Australian aboriginal fire knowledge in the Great Western Woodlands. Austral Ecology, 41(7), 716–732. https://doi.org/10.1111/aec.12377
Rother, M. T., Huffman, J. M., Guiterman, C. H., Robertson, K. M., & Jones, N. (2020). A history of recurrent, low-severity fire without fire exclusion in southeastern pine savannas, USA. Forest Ecology and Management, 475, 118406. https://doi.org/10.1016/j.foreco.2020.118406
Western Australian Herbarium, Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions. (n.d.). Florabase-the Western Australian flora. https://florabase.dbca.wa.gov.au/search/advanced?genus=drosera
Christopher Nolte
Using Genetics to Get a Glimpse into the Past
Three generations of a family are at the coast fishing, each with a different perspective of the current conditions, shaped by the environment they have come to know. The grandparent recalls when one could go out for an afternoon and catch a week’s worth of food with ease. The parent, hearing these stories, witnessed the decline in abundance firsthand. The youngest, familiar only with the current state being lucky to maybe catch one or two fish, is frustrated at the decline since the previous generation not even knowing what the grandparent experienced. Their individual experiences highlight the concept of the shifting baseline syndrome (Pauly 1995). As each generation witnesses a slightly more diminished version of nature, that version becomes their “normal.”
In conservation, this shifting baseline syndrome is important to consider when setting recovery targets. The issue with estimating the number of individuals for populations and species currently is that conservation goals can underrepresent previous abundances. A comparison of distribution range and population size for a particular species over a few decades could indicate that no significant change in habitat or abundance has occurred, but this viewpoint could change if the natural historic range and population size are known. This effect was observed for 12 large mammal species in South Africa, with a significantly higher impact on carnivores (Monsarrat et al. 2019).
Many species go through near-extinction events through their demographic history as a result of stochastic environments. However, recently there has been a greater number of species experiencing declines in population sizes. Species can recover with little impact on their long-term viability, but some are at greater risk due to a loss of genetic variation through genetic drift (random chance of losing some genes in a population). Populations that have undergone severe declines are often characterized by low effective population size (Ne), slow population growth rates, limited gene flow with other populations, and inbreeding. Knowing the Ne is a critical parameter in wildlife management and can provide insight into a species’ demographic history and potential population viability. Understanding both the genetic variation within and among populations is essential for assessing the long-term impacts of human activities on wildlife populations. In this case, studying the genetic makeup of a species or even different populations can help us understand what the previous abundances were.
How do we use genetics to look at population history?
Genetic data can be used to infer past population sizes, growth rates, and migration patterns through models that estimate how genetic diversity changes over time under different scenarios. Various techniques provide estimates of demographic parameters, helping to reconstruct the historical dynamics of populations. The use of genetics to investigate population history has become a popular approach, especially with Next-Generation Sequencing (NGS) technologies. NGS has made sequencing large parts of the genome cheaper and can be more readily applied to nonmodel species that are of conservation concern. At first glance, the idea would be that more genetic diversity means more individuals, and to some degree that would be correct, but there are more nuanced approaches.
In terms of inferring population history using genomics, ancient history is generally referred as an event occurring more than 1000 generations ago. When there are changes to a gene (aka mutation) these are called SNPs (single nucleotide polymorphism), pronounced snips, and are useful for understanding genetic diversity (Figure 1). The frequency and number of these mutations can help determine the history of each population.
The analysis of the frequency and distribution of these SNPs across a genome can reveal significant insights into the demographic history of populations. For instance, a balanced distribution of SNPs across different frequency sites can signal that the population has experienced a bottleneck event (Figure 1 B). Such an event is characterized by a sharp reduction in population size, followed by a rapid expansion, leading to a loss of genetic variation and a uniform distribution of SNPs. This can occur due to various factors, including environmental catastrophes, diseases, or human activities, which drastically reduce the population’s size, affecting its genetic structure.
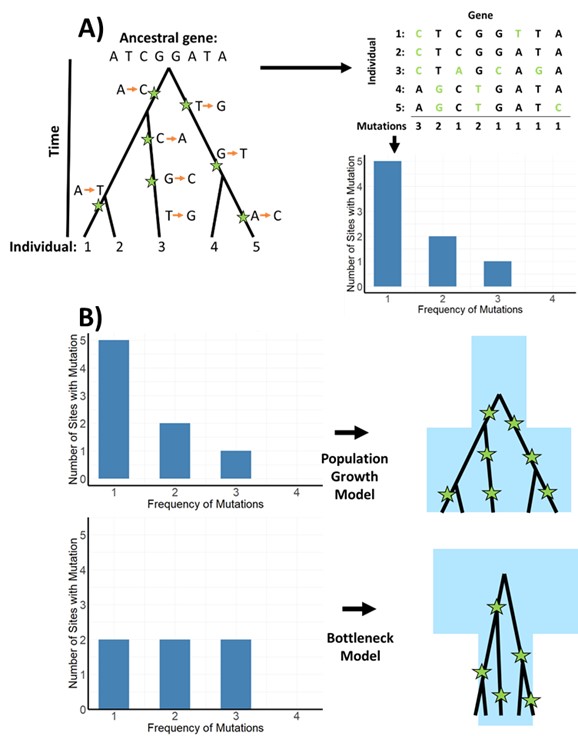
Figure 1: Where mutations occur on a genealogy can help show genetic variation. Using the frequency and number of sites with mutations helps determine population history. A) How single nucleotide polymorphisms are identified and the frequency of the are calculated, the orange arrows indicate the base change on the genome. B) Using the frequency of these mutations one can determine population history, in this example a low number of even mutations indicates shows a bottleneck event (adapted from Beichman et al 2018)
Investigating population history through genetics is important in conservation, particularly when addressing the issue of the shifting baseline syndrome. Genetic studies of population histories offer a tool to counteract this baseline by providing evidence of historical biodiversity and population abundances, enabling a more accurate assessment of the extent of human impact over time. The shifting baseline syndrome leads to a gradual lowering of expectations for ecosystem health and biodiversity, with each generation accepting a degraded environment as the new ‘normal’. By providing a long-term view of population histories and diversity, genetics offers a way to address to this syndrome. Using genomics can help show the magnitude of biodiversity loss and environmental change, allowing a greater appreciation for conservation efforts needed to restore ecosystems or species to their former state or to maintain current diversity.
References
Pauly, D. (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends in ecology & evolution, 10(10), 430.
Monsarrat, S., Novellie, P., Rushworth, I., & Kerley, G. (2019). Shifted distribution baselines. Philosophical Transactions: Biological Sciences, 374(1788), 1-11.
Beichman, A. C., Huerta-Sanchez, E., & Lohmueller, K. E. (2018). Using genomic data to infer historic population dynamics of nonmodel organisms. Annual Review of Ecology, Evolution, and Systematics, 49, 433-456.
Sikander Khare
The Biodiversity-Productivity Relationship in Natural Forests: Evidence and Theory
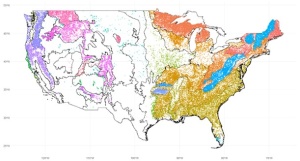
Forest Inventory & Analysis (FIA) plots in naturally assembled forest communities colored according to their associated FIA ecoprovince. Ecoprovince boundaries are also shown.
Forests represent 65% of the world’s biomass (Bar-On et al., 2018; Pan et al., 2013), and play a pivotal role in mediating global biogeochemical cycles, like the carbon cycle. Trees produce biomass by taking up carbon from the atmosphere, so preserving forest biomass production is seen as an essential nature-based solution to climate change. Until the 90s, community ecology had traditionally focused on the processes that generate and maintain diversity but had competing and contradictory theories about its functional consequences, and ecosystem ecologists largely ignored biodiversity. As a response to increasing threats to biodiversity, ecologists began asking how diversity affects the functioning of ecosystems and set up several experiments across the world to investigate this. The most famous biodiversity-ecosystem (BEF) experiments, Cedar Creek (Tilman, Knops, et al., 1997), BIODEPTH (Hector et al., 1999), and Jena (Weisser et al., 2017), consist of small-scale experimental studies of herbaceous plants and suggest that greater plant species richness increases net primary productivity. There are important differences between herbaceous plants and trees. Grassland studies run several lifetimes whereas forest studies run for less than the lifetime of an average trees. As a result, grasslands have higher levels of mortality, recruitment, and species turnover, influencing species proportions, evenness, and age structure. Another important difference is the importance of stand structure in forest communities (Forrester & Bauhus, 2016).
In experimental forest plots, the evidence points to a positive biodiversity-productivity relationship. One global meta-analysis of 54 experimental forest biodiversity-productivity studies concluded that polycultures have 24% higher productivity than monocultures (Zhang et al., 2012), and another global meta-analysis of 126 studies found that mixed-species stands have 15% higher productivity than single-species stands (Jactel et al., 2018). Most experimental forest biodiversity-productivity relationships are positive but saturate (Zhang et al., 2012). Saturating biodiversity-productivity relationships are also observed in grassland communities (Tilman et al., 2001) and are predicted from theoretical principles (Tilman, Lehman, et al., 1997).
However, results from experimental BEF studies do not necessarily transfer to natural systems (Wardle, 2016). Although there is no meta-analysis of observational forest biodiversity-productivity studies, they in general point to a positive to neutral relationship (van der Plas, 2019). Vilà et al. (2013) found results in European forest inventory plots that agree with experimental studies. Liang et al. (2016) found a positive saturating BPR in global inventory plots, but their analysis has been heavily criticized (Chisholm & Dutta Gupta, 2023). Other studies have found near-zero, non-significant, or mixed slopes for forest biodiversity-productivity relationships (Fei et al., 2018; Finegan et al., 2015). An influential global study of observational grassland plots found a strong and consistent enhancement of productivity by species richness (Grace et al., 2016) using structural equation modeling (SEM) to control for confounding factors and test existing theory. In my project, I am attempting to use a similar method to quantify the relationship between tree diversity and productivity from tens of thousands of observation forest inventory plots all across the US (pictured above), which span enormous environmental gradients. One of the fundamental assumptions of SEMs are the presence and directions of causal pathways between variables, both of which can only be justified through theoretical ideas and existing evidence (Grace et al., 2015). Below, I will briefly review some of the theory and experimental evidence for the BEF relationship in forests.
Biodiversity has a positive effect on productivity when mixed species stands display overyieliding, meaning on average they are more productive than the average single species stand. Generally, two basic mechanisms have been posited to explain the positive relationship between diversity and productivity:
- Species selection: more diverse stands are more productive because they are more likely to contain highly productive species.
- Niche complementarity: more diverse stands contain species that are functionally complementary, leading to higher productivity than what would be expected from their productivity in monospecific stands. Niche complementarity itself is often subdivided into two mechanisms that often occur together and are difficult to disentangle in practice (Ammer, 2019; Forrester & Bauhus, 2016):
- Facilitation: occurs when one species improves resource availability or climatic/biotic conditions for other species and it can be driven by a range of biotic and abiotic causes. Some relevant examples in forests include nitrogen fixation, mycorrhizal associations, hydraulic lift, and improved microclimatic conditions that lead to the prevention of frost (Chisholm & Dutta Gupta, 2023; Forrester & Bauhus, 2016).
- Competition reduction: occurs when intraspecific competition is replaced with less intense interspecific competition. In forests, temporal, spatial, and chemical stratification within roots systems and canopies are an important form of competition reduction (Forrester & Bauhus, 2016). Greater differentiation of shade tolerance niches and tree crown architecture may also lead to greater light interception and greater productivity (Chisholm & Dutta Gupta, 2023).
Loreau and Hector (2001) developed a method to quantify and decompose the relative contribution of these two mechanisms to overyielding in experimental stands of herbaceous plants. However, such methods are not applicable to observational data (J. W. Fox, 2005). One potential way to test the relative contribution of these mechanisms could be through the use of functional trait data. From trait-based perspective, the complementarity effect is characterized by trait variation that enhances collective performance, while the selection effect favors species with traits that may lead to increased productivity (Ammer, 2019). Poorter et al. (2017) used SEMs to compare the relative effect of community weighted mean (CWM) traits vs. taxonomic diversity on biomass dynamics. A stronger effect of CWMs would support the biomass-ratio hypothesis (similar to selection effect), while a stronger effect of taxonomic diversity would imply greater niche complementarity. van der plas (2019) also distinguishes between the effect of biodiversity, defined as genetic, taxonomic, phylogenetic, and functional diversity, versus the effect functional composition, which includes presence or relative abundance of a certain species or functional group (e.g. legumes), a combination of species, or CWM trait values (e.g. leaf nitrogen content). The presence of nitrogen fixers can explain some or all of the positive effect of biodiversity on productivity observed in experimental forest studies (Hulvey et al., 2013).
The strength of the biodiversity-productivity relationship is supposed become stronger with stand age and vary along stress gradients. Transgressive overyielding is when mixed stands are more productive than the most productive monoculture and is a good indication of a strong BEF relationship. In experimental forest plots, Jactel et al. (2018) found that the effect size for transgressive overyielding was slightly negative and non-significant, and Hulvey et al. (2013) found a reduced positive effect size compared to overyielding. In experimental herbaceous communities, transgressive overyielding has only been revealed with longer-term data (Cardinale et al., 2007). A meta-analysis of these studies showed that niche complementarity can be as important as species selection and that the importance of niche complementarity increases over time (Cardinale et al., 2007). Complementarity effects may become stronger over time because of selection for increased niche differentiation between species (Zuppinger-Dingley et al., 2014), resulting in increased functional trait diversity. Deteriorating productivity in low diversity treatments (including monocultures) may be due to negative plant–soil pathogen feedbacks (Maron et al., 2011). It may be the case that existing experimental and observational forest plots are not old enough to observe transgressive overyielding, especially given the increased lifetime of trees compared to herbaceous plants. The stress gradient hypothesis (SGH) posits that facilitation will increase and competition will decrease as environmental conditions become harsher (Bertness & Callaway, 1994). Some empirical evidence in forests support this prediction (Jucker et al., 2016; Paquette & Messier, 2011), but others point in the opposite direction (Jactel et al., 2018). Forrester et al. (2014) argue that SGH studies confound species diversity with stand density, and that contrary to the SGH, positive relationships between complementarity and growing conditions were found in about 25 % of forest studies in a literature review.
In the next blog post, I will discuss the various factors that may confound the relationship between diversity and productivity in observational plots of naturally assembled forest communities.
References
Ammer, C. (2019). Diversity and forest productivity in a changing climate. New Phytologist, 221(1), 50–66. https://doi.org/10.1111/nph.15263
Bar-On, Y. M., Phillips, R., & Milo, R. (2018). The biomass distribution on Earth. Proceedings of the National Academy of Sciences, 115(25), 6506–6511. https://doi.org/10.1073/pnas.1711842115
Bertness, M. D., & Callaway, R. (1994). Positive interactions in communities. Trends in Ecology & Evolution, 9(5), 191–193. https://doi.org/10.1016/0169-5347(94)90088-4
Cardinale, B. J., Wright, J. P., Cadotte, M. W., Carroll, I. T., Hector, A., Srivastava, D. S., Loreau, M., & Weis, J. J. (2007). Impacts of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences, 104(46), 18123–18128. https://doi.org/10.1073/pnas.0709069104
Chisholm, R. A., & Dutta Gupta, T. (2023). A critical assessment of the biodiversity–productivity relationship in forests and implications for conservation. Oecologia, 201(4), 887–900. https://doi.org/10.1007/s00442-023-05363-4
Fei, S., Jo, I., Guo, Q., Wardle, D. A., Fang, J., Chen, A., Oswalt, C. M., & Brockerhoff, E. G. (2018). Impacts of climate on the biodiversity-productivity relationship in natural forests. Nature Communications, 9(1), 5436. https://doi.org/10.1038/s41467-018-07880-w
Finegan, B., Peña-Claros, M., de Oliveira, A., Ascarrunz, N., Bret-Harte, M. S., Carreño-Rocabado, G., Casanoves, F., Díaz, S., Eguiguren Velepucha, P., Fernandez, F., Licona, J. C., Lorenzo, L., Salgado Negret, B., Vaz, M., & Poorter, L. (2015). Does functional trait diversity predict above-ground biomass and productivity of tropical forests? Testing three alternative hypotheses. Journal of Ecology, 103(1), 191–201. https://doi.org/10.1111/1365-2745.12346
Forrester, D. I. (2014). The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. Forest Ecology and Management, 312, 282–292. https://doi.org/10.1016/j.foreco.2013.10.003
Forrester, D. I., & Bauhus, J. (2016). A Review of Processes Behind Diversity—Productivity Relationships in Forests. Current Forestry Reports, 2(1), 45–61. https://doi.org/10.1007/s40725-016-0031-2
Fox, J. W. (2005). Interpreting the “selection effect” of biodiversity on ecosystem function. Ecology Letters, 8(8), 846–856. https://doi.org/10.1111/j.1461-0248.2005.00795.x
Grace, J. B., Anderson, T. M., Seabloom, E. W., Borer, E. T., Adler, P. B., Harpole, W. S., Hautier, Y., Hillebrand, H., Lind, E. M., Pärtel, M., Bakker, J. D., Buckley, Y. M., Crawley, M. J., Damschen, E. I., Davies, K. F., Fay, P. A., Firn, J., Gruner, D. S., Hector, A., … Smith, M. D. (2016). Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature, 529(7586), 390–393. https://doi.org/10.1038/nature16524
Grace, J. B., Scheiner, S. M. `, & Schoolmaster, D. R. Jr. (2015). Structural equation modeling: building and evaluating causal models. In G. A. Fox, S. Negrete-Yankelevich, & V. J. Sosa (Eds.), Ecological Statistics: Contemporary Theory and Application (pp. 168–199). Oxford University Press.
Hector, A., Schmid, B., Beierkuhnlein, C., Caldeira, M. C., Diemer, M., Dimitrakopoulos, P. G., Finn, J. A., Freitas, H., Giller, P. S., Good, J., Harris, R., Högberg, P., Huss-Danell, K., Joshi, J., Jumpponen, A., Körner, C., Leadley, P. W., Loreau, M., Minns, A., … Lawton, J. H. (1999). Plant Diversity and Productivity Experiments in European Grasslands. Science, 286(5442), 1123–1127. https://doi.org/10.1126/science.286.5442.1123
Hulvey, K. B., Hobbs, R. J., Standish, R. J., Lindenmayer, D. B., Lach, L., & Perring, M. P. (2013). Benefits of tree mixes in carbon plantings. Nature Climate Change, 3(10), 869–874. https://doi.org/10.1038/nclimate1862
Jactel, H., Gritti, E. S., Drössler, L., Forrester, D. I., Mason, W. L., Morin, X., Pretzsch, H., & Castagneyrol, B. (2018). Positive biodiversity–productivity relationships in forests: climate matters. Biology Letters, 14(4), 20170747. https://doi.org/10.1098/rsbl.2017.0747
Jucker, T., Avăcăriței, D., Bărnoaiea, I., Duduman, G., Bouriaud, O., & Coomes, D. A. (2016). Climate modulates the effects of tree diversity on forest productivity. Journal of Ecology, 104(2), 388–398. https://doi.org/10.1111/1365-2745.12522
Liang, J., Crowther, T. W., Picard, N., Wiser, S., Zhou, M., Alberti, G., Schulze, E.-D., McGuire, A. D., Bozzato, F., Pretzsch, H., de-Miguel, S., Paquette, A., Hérault, B., Scherer-Lorenzen, M., Barrett, C. B., Glick, H. B., Hengeveld, G. M., Nabuurs, G.-J., Pfautsch, S., … Reich, P. B. (2016). Positive biodiversity-productivity relationship predominant in global forests. Science, 354(6309). https://doi.org/10.1126/science.aaf8957
Loreau, M., & Hector, A. (2001). Partitioning selection and complementarity in biodiversity experiments. Nature, 412(6842), 72–76. https://doi.org/10.1038/35083573
Maron, J. L., Marler, M., Klironomos, J. N., & Cleveland, C. C. (2011). Soil fungal pathogens and the relationship between plant diversity and productivity. Ecology Letters, 14(1), 36–41. https://doi.org/10.1111/j.1461-0248.2010.01547.x
Pan, Y., Birdsey, R. A., Phillips, O. L., & Jackson, R. B. (2013). The Structure, Distribution, and Biomass of the World’s Forests. Annual Review of Ecology, Evolution, and Systematics, 44(1), 593–622. https://doi.org/10.1146/annurev-ecolsys-110512-135914
Paquette, A., & Messier, C. (2011). The effect of biodiversity on tree productivity: from temperate to boreal forests. Global Ecology and Biogeography, 20(1), 170–180. https://doi.org/10.1111/j.1466-8238.2010.00592.x
Poorter, L., van der Sande, M. T., Arets, E. J. M. M., Ascarrunz, N., Enquist, B. J., Finegan, B., Licona, J. C., Martínez‐Ramos, M., Mazzei, L., Meave, J. A., Muñoz, R., Nytch, C. J., de Oliveira, A. A., Pérez‐García, E. A., Prado‐Junior, J., Rodríguez‐Velázques, J., Ruschel, A. R., Salgado‐Negret, B., Schiavini, I., … Peña‐Claros, M. (2017). Biodiversity and climate determine the functioning of Neotropical forests. Global Ecology and Biogeography, 26(12), 1423–1434. https://doi.org/10.1111/geb.12668
Tilman, D., Knops, J., Wedin, D., Reich, P., Ritchie, M., & Siemann, E. (1997). The Influence of Functional Diversity and Composition on Ecosystem Processes. Science, 277(5330), 1300–1302. https://doi.org/10.1126/science.277.5330.1300
Tilman, D., Lehman, C. L., & Thomson, K. T. (1997). Plant diversity and ecosystem productivity: Theoretical considerations. Proceedings of the National Academy of Sciences, 94(5), 1857–1861. https://doi.org/10.1073/pnas.94.5.1857
Tilman, D., Reich, P. B., Knops, J., Wedin, D., Mielke, T., & Lehman, C. (2001). Diversity and Productivity in a Long-Term Grassland Experiment. Science, 294(5543), 843–845. https://doi.org/10.1126/science.1060391
van der Plas, F. (2019). Biodiversity and ecosystem functioning in naturally assembled communities. Biological Reviews, brv.12499. https://doi.org/10.1111/brv.12499
Vilà, M., Carrillo-Gavilán, A., Vayreda, J., Bugmann, H., Fridman, J., Grodzki, W., Haase, J., Kunstler, G., Schelhaas, M., & Trasobares, A. (2013). Disentangling Biodiversity and Climatic Determinants of Wood Production. PLoS ONE, 8(2), e53530. https://doi.org/10.1371/journal.pone.0053530
Wardle, D. A. (2016). Do experiments exploring plant diversity–ecosystem functioning relationships inform how biodiversity loss impacts natural ecosystems? Journal of Vegetation Science, 27(3), 646–653. https://doi.org/10.1111/jvs.12399
Weisser, W. W., Roscher, C., Meyer, S. T., Ebeling, A., Luo, G., Allan, E., Beßler, H., Barnard, R. L., Buchmann, N., Buscot, F., Engels, C., Fischer, C., Fischer, M., Gessler, A., Gleixner, G., Halle, S., Hildebrandt, A., Hillebrand, H., de Kroon, H., … Eisenhauer, N. (2017). Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic and Applied Ecology, 23, 1–73. https://doi.org/10.1016/j.baae.2017.06.002
Zhang, Y., Chen, H. Y. H., & Reich, P. B. (2012). Forest productivity increases with evenness, species richness and trait variation: a global meta-analysis. Journal of Ecology, 100(3), 742–749. https://doi.org/10.1111/j.1365-2745.2011.01944.x
Zuppinger-Dingley, D., Schmid, B., Petermann, J. S., Yadav, V., De Deyn, G. B., & Flynn, D. F. B. (2014). Selection for niche differentiation in plant communities increases biodiversity effects. Nature, 515(7525), 108–111. https://doi.org/10.1038/nature13869
Vanessa Luna Celino
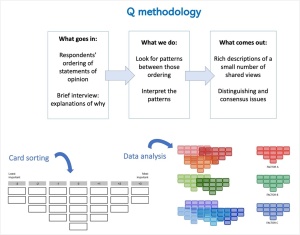
Sorting Cards in Social-Environmental Research: Sharing Experiences about Q Methodology
Q methodology combines qualitative and quantitative techniques to study subjectivity. Using Q techniques, researchers are able to uncover and interpret shared views, perspectives, opinions, values, and beliefs (Brown, 1996). It provides a systematic and rigorous method for examining human subjectivity that helps identify and understand similarities and differences in individual positions through their ranking of a set of fixed statements (Addams & Proops, 2000; Brown, 1996; Dasgupta & Vira, 2005; Ramlo, 2016).
In April 2024, Silvia de Melo Futada (PhD student, SNRE) and I organized a workshop on Q methodology, where participants learned from the experiences of three UF researchers applying it in their doctoral projects: Dr. Sinomar F. Fonseca Jr (SNRE), Dr. Felipe Veluk Gutierrez (SFFGS), and myself. Dr. Fonseca utilized Q methodology to assess local stakeholder perspectives regarding the free, prior, and informed consent processes related to significant infrastructure projects. Dr. Veluk Gutierrez employed this approach to gauge stakeholder attitudes towards Amazonian nut community-based initiatives and bioeconomy. Both Sinomar and Felipe conducted their research in the Brazilian Amazon. My research, on the other hand, utilized Q methodology to engage local stakeholders, including small-scale farmers, in discussions concerning the role of fire and its effective management in the Peruvian Andes.
During the workshop, we requested participants to form pairs and practice data collection of this method. One person gave instructions while the other sorted 23 statements (or actions) related to fire management -the same I used in my doctoral research- into a grid. Participants acting as respondents sorted the statements in two stages. The first stage involved arranging the statements in three piles, considering the level of effectivity of each statement: most effective, least effective, and neutral. Next came the actual sorting, on which respondents considered how strongly they felt about each statement. They placed each one along a response grid or Q sort structure provided in
the workshop. The Q sort resembled a normal distribution or bell curve, with most statements placed in the middle or neutral area and fewer at the extremes. Since respondents ranked statements relative to each other, they adhered to the prescribed Q sort structure when arranging the statements. The resulting Q sort or grid arrangement was entirely subjective, reflecting the respondent’s individual views or opinions.
Further data analysis involves correlation and factor analysis, with each Q sort serving as the unit of analysis. Unlike other methodologies that compare group responses, Q methodology assesses all collected Q sorts against each other. Pattern analysis identifies similar Q sorts, forming clusters representing shared views or subjectivity, leading to the emergence of factors representing commonly held viewpoints. Factor analysis then generates composite Q sorts for each factor, providing a representative arrangement of statements. These composite Q sorts, coupled with transcripts of participant interviews, yield detailed explanations of shared viewpoints. The process is reductive, beginning with numerous viewpoints and culminating in a concise selection of key perspectives. In the end, Q methodology does not provide generalizable results to an entire population because it relies on purposeful samples, but it does give an approximation of the diversity of viewpoints existing in a particular set of key actors.
In my research on fire management, I conducted Q methodology with 56 stakeholders, including members of Quechua communities, firefighters, researchers, nonprofit organizations, protected areas, and government agents. Factor analysis revealed three distinct viewpoints on the role of fire, ranging from emphasizing the negative impacts of fire on ecosystem services (e.g., on biodiversity and climate change impacts) to acknowledging some of the benefits of fire for rural wellbeing (e.g., that agricultural burns open new farmland or fire as the most accessible way to work the farm). My research also found three viewpoints on effective fire management: top-down fire suppression, community-based fire suppression, and community-based fire management. Most government agents and firefighters, holding an emphasis on the negative impacts of escaped fires, advocated for the continuation of top-down fire suppression policies, whereas community members and other external actors with a more balanced perception of fire advocated for community-based actions, either suppression or management. Q methodology served as a tool to reflect on the need for fire governance in the Peruvian Andes that reconciles the different needs of various stakeholders, especially those of frontline fire users.
Literature cited:
Addams, H., & Proops, J. L. (2000). Social discourse and environmental policy: an application of Q methodology (Vol. 16). Edward Elgar Publishing.
Brown, S. R. (1996). Q Methodology and Qualitative Research. Qualitative health research, 6(4), 561-567. https://doi.org/10.1177/104973239600600408
Dasgupta, P., & Vira, B. (2005). Q methodology for mapping stakeholder perceptions in participatory forest management. Annex B3 of the Final Technical Report of project, 8280.
Ramlo, S. (2016). Mixed Method Lessons Learned From 80 Years of Q Methodology. Journal of mixed methods research, 10(1), 28-45. https://doi.org/10.1177/1558689815610998
Akshay Vinod Anand
Walking in the Footsteps of Historical “Birders”
“I pelted the bush with stones until I flushed out all individuals of the Gray-headed Bulbul. I recorded three individuals flying to nearby cover.” This is an excerpt from an article published in the early 1900’s in the Journal of the Bombay Natural History Society (JBNHS). I leaned back in my chair and thought to myself “imagine if this had become a standardized sampling protocol for birds…”
A. Akshay et al., 2024: “We used the standardized sampling method of stone pelting to survey birds. At each sampling location all trees and shrubs within a 50-meter radius were pelted with 50-gram stones acquired from Wildlife Rocks Inc. All birds scurrying for shelter from our barrage of stones were recorded along with their distance from the stone pelter.” This hardly reads well and is definitely not the most ethical way to survey birds.
The field of ecology and wildlife conservation has come a long way since the 1900’s.
So, why was I scouring through literature published in the 18 and 1900’s, questioning the ethical foundations on which field sampling in the field of ecology and wildlife conservation were built on? Well, I was part of a team that was planning a historical resurvey of birds in the Nilgiri Mountain range of the Western Ghats of India. Our goal was to understand how landscape and climate change had impacted bird populations in the region over the last 200 years. As with any historical resurvey the first step was to collect historical occurrence records of birds from these ancient mountains. We would then go out to these locations and survey birds, using the more widely accepted and ethical method of observational point counts. I spent the better part of 2020 scrutinizing the earliest issues of the JBNHS to curate a list of sites that we would resurvey. While the task was a bit mundane, I often came across gems, like the excerpt above, that gave me a wonderful insight into the mindset of early birders.
Now why was this resurvey important? When the colonial British rule first set up base in the Nilgiri mountains the landscape was a beautiful mosaic of high elevation grasslands and short-statured Shola forests. Feeling homesick, the British officers posted in the Nilgiris drew similarities between the southern British downs and the Shola forest-grassland mosaic and postings in the Nilgiris were highly sought after. As more and more officers began to settle in these mountains, they also realized that these large swathes of grassland were easily afforested. With this realization began one of the most drastic landcover transformations the country has seen. Commercially valuable timber like acacia, pine and eucalyptus were planted across large portions of the upper plateau of the Nilgiris [1]. These fast-growing exotic species quickly overtook native vegetation, forever changing the landscape. The landcover transformation did not stop there, the officers also realized that these rolling grasslands and the cool climate of the Nilgiris were ideal for growing tea. To ensure they did not need to travel far for the perfect ‘cuppa’ tea the British established massive tea plantations across the Nilgiris. What was once a magnificent mosaic of grasslands and forests has now been converted into a drab degraded landscape of tea and timber. This drastic land transformation and impending climate change pose dire implications for birds in the region, and it was our goal to understand exactly how our feathery friends have been impacted by these anthropogenic pressures.
It was late October by the time we had a curated list of resurvey sites. The rigorous task of historical data collection was over, and it was time for the fun part, fieldwork! I packed my motorcycle with all my field essentials and set off on a 350-mile journey across the country to reach the Nilgiri mountains. For the most part of this ride I passed through farmland and cities, but as I neared the mountains, I understood where these ancient hills got their iconic name from. Translated from Tamil the Nilgiris mean “blue mountains”, as I turned off the highway I saw it in the distance, a deep blue towering silhouette. The Nilgiris in all its splendor. I slowed down and began my winding ascent through these hills. It was fascinating to see the landscape change around me as I ascended. It started with low elevation dry deciduous forests and slowly transformed into moist, semi evergreen forests at the mid-elevations. As I climbed higher, the semi-evergreen gave way to lush, tropical rainforests, and on the peaks and ridges I could see the remnants of the gorgeous Shola forest – grassland mosaic. Mind you, these forest stands were only in small pockets along the slopes of these hills, that were probably too steep to grow tea or timber.
Over the next six months we would resurvey numerous historical sites and assess how bird communities were adapting to the changes in landcover and climate. Every morning, we would set out, in the biting cold, to reach our survey sites before sunrise. And at every site, I couldn’t help thinking about how this landscape might have looked 200 years ago. In 2020 I was standing in a pine forest, but in the early 1900’s was this a vast expanse of grasses or a lush tropical forest? It broke my heart every day, thinking about what once was, and what has become of it. Despite the constant heartache, we did see some beautiful birds, exquisite wildlife and striking scenery that lifted our spirits (Fig 1).

Figure 1: Endemic wildlife from the Western Ghats: (clockwise from the top left) the Nilgiri Flycatcher (Eumyias albicaudatus), Nilgiri Laughingthrush (Montecincla cachinnans), Nilgiri Tahr (Nilgiritragus hylocrius), and the Bronze-headed vine snake (Ahaetulla perroteti).
The cherry on top was when we got to visit the Mukurthi National Park. This National Park is closed to visitors, with only a handful of research expeditions having been conducted in the recent past, and we were part of this privileged few. Mukurthi is home to the last, untouched expanses of the Shola forest – grassland mosaic (cover photo). It was absolutely stunning, rolling hilltops covered in thick grass, with deep ravines engulfed in cauliflower-esque Shola forests. We spent ten days in the heart of this National Park, hiking through knee high tussocks of grass and crawling through 4-meter high, mature, Shola forests to reach out survey sites. Completely isolated from the outside world, our evenings were spent chatting with the forest rangers who were posted inside the park year-round. Their stories, experiences and knowledge of this landscape was the highlight of our visit. We eventually learned that the hiking shed we were staying in was the exact same one that the father of Indian ornithology, Dr. Salim Ali, had stayed in during his survey of the Nilgiris. It was a surreal realization; I knew then that I was truly walking in the footsteps of historical birders.
References
[1] M. Arasumani, D. Khan, C. K. Vishnudas, M. Muthukumar, M. Bunyan, and V. V. Robin, “Invasion compounds an ecosystem-wide loss to afforestation in the tropical grasslands of the Shola Sky Islands,” Biol. Conserv., vol. 230, pp. 141–150, Feb. 2019, doi: 10.1016/j.biocon.2018.12.019.